Abstract
Purpose
The efficiency of ischemic postconditioning (IPC) was evaluated in a rat model of ischemic liver. Concentration of survivin of liver tissue correlated with the degree of antiapoptosis, so survivin was estimated to evaluate the efficiency of IPC on ischemic reperfusion (IR) injury.
Methods
Twenty-four healthy rats were divided to three groups (SHAM, IR, and IPC). Rats in the SHAM group displayed no change during 3 hours. Rats in the IR group were ischemic within 1 hour of clamping the left hepatic artery and left portal vein. Reperfusion for 2 hours was then done. IPC group, intermittent 2, 3, 5, and 7 minutes of reperfusion followed by 1 hour of warm ischemia. Two-minute reocclusion was done after each reperfusion. Rat sera were analyzed for AST and ALT, and Western blot analysis of rat liver tissue of rats evaluated malondialdehyde (MDA) and survivin.
Ischemia is defined as the diminished oxygen supply to levels no longer sufficient to maintain physiologic cell function. Oxygenated blood reperfusion is very important to the survival of ischemic tissue, but reperfusion can injure ischemic tissue. Reperfusion injury refers to the tissue damage that is caused when the blood supply returns to the tissue after a period of ischemia. The absence of oxygen from blood during the ischemic period creates a condition in which the restoration of circulation results in inflammation and oxidative damage through the induction of oxidative stress rather than restoration of normal function [1].
The mechanism of ischemic reperfusion injury of the liver is complicated and interactive. Oxidative stress is initiated from reactive oxygen species produced by Kupffer cells [2]. Microcirculation in the hepatic sinusoid is inhibited by endothelial swelling, vasospasm, leucocyte entrapment and plate aggregation, and complement activation resulting from ischemic reperfusion injury is fatal. Neutrophil infiltration-induced inflammatory cytokines and oxidative free radicals aggravate liver cell injury. This injury is usually seen during the first 48 hours of reperfusion [3].
Malondialdehyde (MDA) is final product of lipid peroxide that can reflect the degree of ischemic reperfusion injury as a product of ischemic damaged cells [4,5]. The survivin gene belongs to the antiapoptotic protein family and is a well-known pivotal antiapoptotic gene. Higher expression of survivin lessens ischemic reperfusion injury [6].
Clinically, ischemic reperfusion liver injury occurs in situations like systemic shock and recovery from trauma, and in surgical procedures including liver transplantation and hepatectomy. The Pringle maneuver is an intraoperative method that halts blood supply from the hepatic artery and portal vein by clamping the porta-hepatis. The maneuver is done to minimize blood loss during hepatectomy. But, a prolonged Pringle time can result in oxidative stress-mediated liver cell injury, which can ultimately result in liver failure [7]. Warm ischemic reperfusion injury occurs in hepatectomy, systemic shock, toxic liver injury and hepatic venous thrombosis. Cold ischemic reperfusion injury occurs in liver that is preserved at cold temperatures during liver transplantation. Warm ischemic reperfusion liver injury is more severe and irreversible damage and multiple organ failure is more frequent [8].
Ischemic postconditioning (IPC) is method involving the intermittent and repetitive preperfusion and occlusion of blood supply before reperfusion [9]. Slow and intermittent oxygen supply to ischemic tissue could prevent the abrupt onset of free radical on excessive oxygen supply, suppress the intracellular production of oxidative free radicals and induce free radical scavengers [10,11,12,13,14,15].
In this study, IPC was done in a rat model of ischemic reperfusion injury. AST and ALT were checked to evaluate the degree of liver injury after IPC. Tissue MDA was evaluated to assess the degree of mitochondrial injury and expression of tissue survivin was analyzed to determine the degree of antiapoptosis. The goal was to determine the efficiency of IPC.
Twenty-four healthy male rats weighing 250-350 g were fed standard lab chow in accordance with the relevant Korean University animal care criterion. The rats were randomly divided into three groups: a sham group (SHAM) group, ischemic reperfusion (IR) group and ischemic postconditioning group (IPC).
Intraperitoneal ketamine (30 mg/kg) injected as the anesthetic. 3-4 hours of anesthesia could be achieved by the intraperitoneal ketamine injection. Within the time, sampling of blood and liver tissue should be taken before euthanasia. So, our experimental group composed of 1 hour of ischemic time and 2 hours of IR or IPC time. After anesthesia, the abdominal cavity was opened by scissors, the hepatic hilum was exposed and the left hepatic artery and portal vein were identified.
SHAM group was the control group, and the rats were not manipulated during the 3-hour experimental period. Rats in the IR group received 1 hour of clamping and 2 hours of reperfusion of left hepatic artery and left portal vein. IPC group, intermittent 2, 3, 5, and 7 minutes of reperfusion followed by 1 hour of warm ischemia. Two-minute reocclusion was done after each reperfusion. After 4 cycles of reperfusion and reocclusion, persistant reperfusion was done.
After reperfusion, blood was withdrawn from the heart and the tissue was collected from the left lobe of the liver. The liver tissue was kept in liquid nitrogen and serum was analyzed for AST and ALT using an automatic biochemical analyzer.
Half of each liver tissue sample was assayed for MDA concentration using an Oxiselect TBARS assay kit (Cellbiolabs, San Diego, CA, USA). TBA regent from the kit was added to the liver tissue for standardization. The absorption was determined at a wavelength of 532 nm using a spectrophotometric plate reader.
The remainder of each liver tissue was used for survivin protein extraction and Western blot analysis. For extraction of protein, 500 µL of chilled RIPA buffer (ELPIS Biotechnology, Daejeon, Korea) was added, the tissue was centrifuged for 20 minutes at 13,000 rpm and the supernatants recovered. Protein assay reagent (Pierce, Rockford, IL, USA) was used for determination of the concentration of survivin. For Western blot, total protein from the tissue was mixed with Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA, USA) and 20 µg of protein was subjected to sodium dodecyl sulfatepolyacrylamide gel electrophoresis. The resolved proteins were electrophoretically transferred at 4℃ to a Protran nitrocellulose membrane (Whatman, Dassel, Germany). The membrane was blocked with blocking buffer composed of Tris-buffered saline (TBS) and 5% skim milk for 30 minutes prior to an overnight incubation with the specific antibody to either Surviving (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or β-actin (1:5,000 dilution; Sigma-Aldrich, St. Louis, MO, USA). Each membrane was rinsed with wash buffer for 20 minutes and incubated with a 1:2,500 dilution of horseradish peroxidase-conjugated secondary antibody in blocking buffer for 1 hour at room temperature. Each membrane was washed twice with TBS containing 0.2% Tween-20. The ECL Plus Western blotting detection reagent (Amersham Biosciences, Pitssburgh, PA, USA) was used to visualize immune-reactive protein, with Image-J software (National Institutes of Health, Bethesda, MD, USA) used for optical density analysis.
The data were analyzed by a one-way analysis of variance and P-values < 0.05 was considered significant.
Among the 24 rats, three anesthesia-related deaths occurred: one in the IR group and two in the IPC group. The remaining eight rats in the SHAM group, seven in the IR group and six in the IPC group were enrolled.
There was no significant difference in body weight between the groups; the mean body weight was 275.63 ± 11.48 g in the SHAM group, 306.57 ± 42.49 g in the IR group and 307.50 ± 29.79 g in the IPC group. Serum ALT was significantly higher in the IR group than in the SHAM group (P = 0.003). IPC was also higher in the SHAM group compared to the IR group, but the difference was not significant (P = 0.101). Serum ALT in the IR group was also higher (but not significantly) than in the IPC group (P = 0.101). Serum AST was significantly higher in the IR group than in the SHAM group (P = 0.001). Serum IPC was higher (but not significantly) in the IPC group compared to the SHAM group (P = 0.150). Finally, AST of the IR group was higher (but not significantly) than the IPC group (P = 0.119) (Table 1).
MDA of liver tissue in the IR and IPC groups were significantly higher than in the SHAM group (P = 0.003 and P = 0.008, respectively). In comparison of the IR and IPC groups, the MDA concentration in the IPC group was slightly higher than that in the IR group (P = 1.000) (Table 1).
Western blots revealed that the level of survivin in the IPC group was higher than that in the IR and SHAM groups. At a relative concentration level, IPC was significantly higher than the IR and SHAM groups (40.6235 in SHAM, 49.6187 in IR, and 75.7718 in IPC; P = 0.021 and P = 0.024, respectively) (Fig. 1).
Ischemic reperfusion injury is frequently seen in liver resection, liver transplantation and systemic shock. Injury ranges from loco-regional liver injury to systemic multiorgan failure. In liver transplantation, primary nonfunction is typically evident in 5%-30% of cases. A nonfunctional liver is very dangerous in a critically-ill patient undergoing liver transplantation [16]. The shortage of donor livers in many countries makes the chance for survival for such patients dim. Preventing graft loss due to ischemic reperfusion injury requires a better understanding of the mechanism of the injury.
Ischemic reperfusion results in the generation free radical oxygen and hyperoxygenation of phospholipids by activated Kupffer cells. AST and ALT are good markers of the degree of liver cell injury. Released free radical oxygen induces hyperoxygenation of phospholipids, leading to the disruption of the cellular membrane around the portal vein. As a result, cells undergoing apoptosis or necrosis release ALT and AST from their cytoplasm.
IPC is a technique of intermittent reperfusion of blood flow before full reperfusion [17]. IPC results in decreased intrahepatic regional infarct [18,19]. In this study, significantly elevated AST and ALT in the IR group as compared to the IPC and SHAM groups was an indicator of a well-controlled model of ischemia. Comparison of the IR and IPC groups showed that AST and ALT were marginally higher in the IR and IPC groups. This was indicative of reduced injury to liver cells in the IPC group of rats than in the IR group, and demonstrated the prevention of ischemia-related cell injury.
Free radical oxygen have been documented to have a pivotal role in ischemic reperfusion injury in several studies. Oxidative injury to polyunsaturated fatty acids results from the disruption of biological membranes by hyperoxygenation with free radical oxygen [18,20].
Cell survival depends on the reversibility of mitochondrial damage that results from ischemic insult. Mitochondrial damage is influential in the development of apoptosis and cell necrosis. Free radical oxygen originates from the mitochondria of cells. Hyper oxygenation of phospholipids by excessive free radical oxygen increases mitochondrial membrane permeability by injury to constituent membrane proteins. Structural breakdown of the mitochondria releases cytochrome to the cytoplasm. As a result, increased caspase activity can induce apoptosis or cell necrosis [21].
Estimation of MDA was presently used as a biochemical marker for the evaluation of mitochondrial damage. The MDA level in the IR and IPC groups was significantly higher than that of the SHAM group. The MDA level in liver tissue from the IPC and IR groups was similar. These results are consistent with the view that IPC has no suppressive effect on the hyperoxygenation of phospholipids by free radical oxygen. However, the tissue sampling time may not have been appropriate for estimation of the MDA level. In another study, there was no difference of MDA level after immediate reperfusion, while at 72 hours significant difference of MDA level was observed between the IR group and the control group [22].
Survivin is an antiapoptotic protein. The gene encoding survivin is very important in the regulation of apoptosis. It was therefore pivotal to explore the effect of the expression of survivin protein caused by ischemia reperfusion injury. Survivin was strongly expressed in the IPC group compared to the IR and SHAM groups in Western blot analyses. Relative concentration of survivin in the IPC group was significantly higher than the IR and SHAM groups, indicating marked antiapoptotic activity in the IPC group compared to the IR group. Reduction of apoptosis and cell necrosis by free radical oxygen after reperfusion may have been induced by the high expression of survivin in the IPC rats.
IPC appears as the promising technique to avoid deleterious consequences of ischemic reperfusion injury. After evident and careful study, IPC could be applied to operation such as hepatectomy requiring Pringle's maneuver and liver transplantation. Warm ischemic reperfusion induced irreversible liver damage and multiple organ failure could be minimized by this method.
In summary, the collective results support the view that IPC can reduce apoptosis and cell necrosis after reperfusion, and can eventually minimize liver tissue damage caused by reperfusion injury.
IPC is less active in preventing hyperoxygenation of mitochondrial phospholipids, but is influential in reducing apoptosis and cell necrosis after reperfusion injury. IPC reduces ischemic reperfusion injury by minimizing apoptosis and cell necrosis.
Clinically, IPC could be applied for operations like liver resection and liver transplantation. Clinical trials with IPC after Pringle's maneuver on liver resection or implantation of orthotropic liver on liver transplantation should be undertaken with the goal of preventing ischemic reperfusion. Laboratory experimentation with other species is necessary before clinical trials can be contemplated. The most appropriate time and gap between perfusion and reperfusion need to be determined in laboratory studies.
Figures and Tables
Fig. 1
Protein expression level of survivin in groups by Western blot analysis (A) andrelative concentration (B). a)P > 0.05, compared with the SHAM group. b)P < 0.05, compared with both the SHAM and IR groups. IR, Ischemic reperfusion; IPC, ischemic postconditioning.
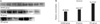
Table 1
Level of total bilirubin, direct bilirubin, ALT and AST in serum and measurement of MDA concentration in hepatic tissue

Values are presented as mean ± standard deviation.
MDA, malondialdehyde; IR, ischemic reperfusion; IPC, ischemic postconditioning.
a)P < 0.05, compared with SHAM group. b)P > 0.05, compared with both SHAM and IR group. c)P < 0.05, compared with both IR and IPC group. d)P > 0.05, compared with IR group.
References
1. Gross GJ, Auchampach JA. Reperfusion injury: does it exist? J Mol Cell Cardiol. 2007; 42:12–18.
2. Nauta RJ, Tsimoyiannis E, Uribe M, Walsh DB, Miller D, Butterfield A. Oxygen-derived free radicals in hepatic ischemia and reperfusion injury in the rat. Surg Gynecol Obstet. 1990; 171:120–125.
3. Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, et al. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007; 9:49–89.
4. Duffy MJ, O'Donovan N, Brennan DJ, Gallagher WM, Ryan BM. Survivin: a promising tumor biomarker. Cancer Lett. 2007; 249:49–60.
5. Ouhtit A, Matrougui K, Bengrine A, Koochekpour S, Zerfaoui M, Yousief Z. Survivin is not only a death encounter but also a survival protein for invading tumor cells. Front Biosci. 2007; 12:1260–1270.
6. Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006; 70:240–253.
7. Lei DX, Peng CH, Peng SY, Jiang XC, Wu YL, Shen HW. Safe upper limit of intermittent hepatic inflow occlusion for liver resection in cirrhotic rats. World J Gastroenterol. 2001; 7:713–717.
8. Wang KX, Hu SY, Jiang XS, Zhu M, Jin B, Zhang GY, et al. Protective effects of ischaemic postconditioning on warm/cold ischaemic reperfusion injury in rat liver: a comparative study with ischaemic preconditioning. Chin Med J (Engl). 2008; 121:2004–2009.
9. Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003; 285:H579–H588.
10. Galagudza M, Kurapeev D, Minasian S, Valen G, Vaage J. Ischemic postconditioning: brief ischemia during reperfusion converts persistent ventricular fibrillation into regular rhythm. Eur J Cardiothorac Surg. 2004; 25:1006–1010.
11. Hausenloy DJ. Signalling pathways in ischaemic postconditioning. Thromb Haemost. 2009; 101:626–634.
12. Minor T, Saad S, Nagelschmidt M, Kotting M, Fu Z, Paul A, et al. Successful transplantation of porcine livers after warm ischemic insult in situ and cold preservation including postconditioning with gaseous oxygen. Transplantation. 1998; 65:1262–1264.
13. Mockford KA, Girn HR, Homer-Vanniasinkam S. Postconditioning: current controversies and clinical implications. Eur J Vasc Endovasc Surg. 2009; 37:437–442.
14. Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusioninduced injury. Cardiovasc Res. 2006; 70:200–211.
15. Zhu XM, Liu XH. Ischemic postconditioning, a new method to protect tissue from ischemia/reperfusion injury. Sheng Li Ke Xue Jin Zhan. 2007; 38:261–265.
16. Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation: a multivariate analysis. Transplantation. 1993; 55:807–813.
17. Wu BQ, Chu WW, Zhang LY, Wang P, Ma QY, Wang DH. Protection of preconditioning, postconditioning and combined therapy against hepatic ischemia/reperfusion injury. Chin J Traumatol. 2007; 10:223–227.
18. Teixeira AR, Molan NT, Kubrusly MS, Bellodi-Privato M, Coelho AM, Leite KR, et al. Postconditioning ameliorates lipid peroxidation in liver ischemia-reperfusion injury in rats. Acta Cir Bras. 2009; 24:52–56.
19. Wang N, Lu JG, He XL, Li N, Qiao Q, Yin JK, et al. Effects of ischemic postconditioning on reperfusion injury in rat liver grafts after orthotopic liver transplantation. Hepatol Res. 2009; 39:382–390.
20. Sola S, Brito MA, Brites D, Moura JJ, Rodrigues CM. Membrane structural changes support the involvement of mitochondria in the bile salt-induced apoptosis of rat hepatocytes. Clin Sci (Lond). 2002; 103:475–485.
21. Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999; 341(Pt 2):233–249.
22. Li JY, Gu X, Yin HZ, Zhou Y, Zhang WH, Qin YM. Protective effect of ischemic preconditioning on hepatic ischemia-reperfusion injury by advancing the expressive phase of survivin in rats. Hepatobiliary Pancreat Dis Int. 2008; 7:615–620.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download