Abstract
Purpose
We applied a long Roux-en-Y (RY) gastrojejunostomy (bypassed jejunum over 100 cm) as a reconstruction method for diabetes control to gastric cancer patients with type 2 diabetes and body mass index (BMI) < 35 kg/m2. The effect of this procedure on diabetes control was assessed.
Methods
We prospectively performed modified RY gastrojejunostmy after curative radical distal gastrectomy. Thirty patients had completed a 1-year follow-up. Patients were followed concerning their diabetic status. The factors included in the investigation were length of bypassed jejunum, BMI and its reduction ratio, glycated hemoglobin (HbA1c), fasting blood glucose, and duration of diabetes. Diabetic status after surgery was assessed in three categories: remission, improvement, and stationary. In evaluation of surgical effects on diabetes control, remission and improvement groups were regarded as effective groups, while stationary was regarded as an ineffective group.
Results
At postoperative one year, statistical significance was observed in the mean BMI and HbA1c. Diabetes control was achieved in 50% of the patients (remission, 30%; improvement, 20%). BMI reduction ratio, preoperative HbA1c, and duration of diabetes were correlated to the status of type 2 diabetes mellitus. The preoperative HbA1c was the most influential predictor in diabetic control.
Conclusion
The effect of long RY gastrojejunostomy after gastrectomy for diabetes control could be contentious but an applicable reconstruction method for diabetes control in gastric cancer patients with type 2 diabetes and BMI < 35 kg/m2. Diabetes remission is expected to be higher in patients with greater BMI reduction, short duration of diabetes, and lower preoperative HbA1c.
Type 2 diabetes mellitus (T2DM) is a global health problem and a progressive chronic disease that can present life-threatening complications. An accepted pathophysiology of T2DM is insulin resistance in muscle and liver, and beta cell failure. However, details of the etiology remain unknown. It is frequently associated with obesity and is difficult to control by current medical treatments including diet, drug therapy and behavior modification. T2DM remission has been observed as an additional outcome of surgical treatment of patients who are morbidly obese. Thus, gastrointestinal metabolic surgery has been established for the treatment of inadequately controlled T2DM patients with a body mass index (BMI) ≥ 35 kg/m2 and has more recently been proposed as a treatment modality for T2DM patients with BMI < 35 kg/m2 [1,2].
Several gastrointestinal metabolic surgeries that were primarily designated to treat morbid obesity result in T2DM remission. Two procedures, the Roux-en-Y (RY) gastric bypass and the biliopancreatic diversion, are more effective treatments for diabetes than other procedures. The physiological basis of the improvement in diabetes after these operations is still uncertain; however, the core hypothesis involves changes in several peptides from the small intestine, which may influence insulin sensitivity and/or insulin secretion.
Usually, the length of bypassed proximal jejunum is about 70-90 cm (biliopancreatic limb, 30-40 cm; alimentary limb, 40-50 cm) in RY reconstruction after distal gastrectomy for gastric cancer. Herein we applied a similar procedure of RY gastric bypass performed in bariatric surgery to curable gastric cancer patients with T2DM for treatment of diabetes in one operation simultaneous with radical surgery for gastric cancer. The aim of the current study was to investigate the efficacy of our modified RY gastrojejunostomy on diabetes remission.
The present study was a single center study. From 2007 to April 2012, thirty gastric cancer patients with T2DM who underwent radical gastrectomy with long RY gastrojejunostomy (open method, n = 18; laparoscopic method, n = 12) were followed at outpatient surgical clinic. Adjuvant chemotherapy was performed for stage II-IV (n = 7) after curative surgery for cancer at medical oncology clinics, and patients of mostly stage I (n = 23) cancer without significant risk of recurrence were followed at outpatient surgical clinics. The following factors were investigated: gender, age, length of bypassed jejunum, preoperative BMI, postoperative BMI, BMI reduction ratio, preoperative insulin or oral diabetic medicine requirement, preoperative and postoperative glycated hemoglobin (HbA1c), duration of diabetes and current status of diabetes. The current status of diabetes was categorized in three groups. Remission was defined as a case where the patients became euglycemic or a HbA1c level was maintained below 6.5% without the use of diabetes medication after surgery. Improvement was defined as a case where the patient became euglycemic or HbA1c level was maintained below 7.0% without the use of diabetic medication or HbA1c level was maintained below 6.5% with the lower dosage of diabetic medication. Stationary was defined as a case in which no change occurred in fasting glucose, HbA1c level and medication after surgery. The usefulness of our modified surgical procedure was grouped into two categories. Clinically, patients were classified in the effective group if the status of diabetes were remission or improvement, and the ineffective group if the status of diabetes was stationary. The recurrence of malignancy had been ruled out through regular follow-up examination including laboratory tests, abdominal computed tomography scan, PET scan and esophagogastroduodenoscopy.
Statistical analysis of data was performed with the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as means and standard deviation. All data were analyzed using chi-square test, independent and paired t-test, Mann-Whitney U test and logistic regression analysis. A P-value of <0.05 was considered significant. This study was approved by the Institutional Review Board of Hallym University Hospital and written consent was obtained from all patients.
Long RY gastrojejunostomy was defined as bypassed proximal jejunum over 100 cm. After the standard radical distal gastrectomy (resection of 70%-80% of stomach), the jejunum was divided 30-120 cm distal to the ligament of Treitz (biliopancreatic limb). The distal limb of the jejunum was anastomosed to the remnant stomach and jejunojejunostomy was undertaken 60-150 cm distal to the gastrojejunostomy (alimentary limb) (Fig. 1).
The characteristics of the subjects are summarized in Table 1. The temporal changes of parameters associated with T2DM are summarized in Table 2. During follow-up, internal herniation or significant nutritional deficiency related to long RY gastrojejunostomy procedure was not found. At postoperative 6 months, 13 of 30 patients (43.3%) were classified in the remission group (HbA1c < 6.5%, without use of diabetic medication) and eight patients (26.7%) showed improved metabolic conditions (improvement group, HbA1c < 7.0%, without use of medication or HbA1c < 6.5%, with lower dosage of medication). However, nine patients (30%) did not change their status of diabetes (stationary group). At postoperative one year, nine patients (30%) were in the remission group, six patients (20%) were in the improvement group and 15 patients (50%) were in the stationary group. When the remission group and improvement group were considered to have clinical effectiveness after surgery, 21 patients (70%) and 15 patients (50%) were categorized in the effective group at postoperative 6 months and one year, respectively. One year after surgery, the mean BMI decreased from 26.8 ± 3.5 to 22.6 ± 2.9 kg/m2, and the mean HbA1c decreased from 7.6% ± 1.6% to 6.9% ± 1.3% with statistical significance. The mean fasting blood glucose decreased from 144.7 ± 38.1 to 136.6 ± 31.8 g/dL without statistical significance (Table 2). Univariable analyses were performed to determine the impact of factors contributing to the amelioration of diabetes mellitus. Age, gender, preoperative BMI, preoperative fasting blood glucose, length of alimentary limb and total length of bypassed jejunum were not significant predictors of diabetes remission or improvement at postoperative one year. Independent t-test with Mann-Whitney U test showed significant differences in preoperative HbA1c, duration of DM, BMI reduction ratio and length of biliopancreatic limb between the effective group and ineffective group (P = 0.017, P = 0.018, P = 0.004, and P = 0.049, respectively) (Table 3). Multivariable analysis using logistic regression test was performed to determine significant predictors affecting the course of DM. In multivariable analysis, preoperative HbA1c level was significantly related amelioration of diabetes and absolute value of contributing degree of preoperative HbA1c was greatest (Table 4). Among 21 patients classified in the effective group (remission group + improvement group) at postoperative 6 months, six patients were classified in the ineffective group (stationary group) at postoperative one year (exacerbation group). Comparison between exacerbated patients (six patients, exacerbation group) and maintaining patients (15 patients, maintenance group) was carried out to investigate significant factors affecting aggravation of diabetes. The changes of clinical parameters associated with DM in exacerbated patients are summarized in Table 5. Statistically significance among two groups were not found for age, gender, preoperative HbA1c, preoperative BMI, preoperative fasting blood glucose, BMI reduction ratio, length of bypassed jejunum and duration of DM (Table 6).
In its' position statement, the International Diabetes Federation wrote that surgery should be an accepted option in patients who have T2DM and BMI ≥ 35 kg/m2 [3]. Surgery should be considered as an alternative management option in patients with BMI 30 to 35 kg/m2 when diabetes cannot be adequately controlled by optimal life style modification and pharmacotherapy, especially in the presence of other major cardiovascular risk factors. Several reports about T2DM patients with severe obesity undergoing gastrointestinal metabolic surgery have demonstrated improvement of DM control [1,4,5]. However, bariatric surgery as a treatment for T2DM in mildly obese (BMI < 35 kg/m2) or nonobese patients has more recently become of matter of intense debate. While relevant studies are few, there is mounting evidence for similar benefits of gastrointestinal metabolic surgery on diabetes in patients with BMI < 35 kg/m2 [6,7,8]. Various b ariatric surgery approaches have been proposed in the literature for the treatment of T2DM in mildly obese and non-obese patients. Depaula et al. [9] in 2008 reported 39 lower BMI patients (BMI < 35 kg/m2) with T2DM treated with a laparoscopic ileal interposition with sleeve gastrectomy; 86.9% of the patients improved in glycemic control. Lee et al. [10] in 2010 published their experience in laparoscopic sleeve gastrectomy in 20 patients with a mean BMI 31.0 kg/m2 who obtained a 50% remission in diabetes and also published a prospective randomized study in 60 diabetic patients with a mean BMI of 30.3 kg/m2. They performed gastric bypass in 30 patients and sleeve gastrectomy in the other 30 patients, obtaining 93% remission for gastric bypass and 47% for sleeve gastrectomy at the one-year follow-up [11]. Rubino and Marescaux [12] performed a study with nonobese diabetic rats that underwent duodenal-jejunal exclusion, and chronicled a significant glucose decrease in about 40%. Since then a few studies about clinical efficacy of duodenal-jejunal bypass surgery for type 2 diabetic control have been reported. Cohen et al. [13] demonstrated resolution of diabetes in two nonobese diabetic patients following duodenal-jejunal bypass surgery. Ramos et al. [14] reported on 20 patients (BMI < 35 kg/m2) who underwent duodenal-jejunal bypass, with a follow up of 6 months, having discontinued their preoperative oral hypoglycemics or insulin or both in 90%. Similarly, Ferzli et al. [15] and Geloneze et al. [16] also concluded that duodenal-jejunal bypass is a safe surgery and a valuable option in the treatment of T2DM. A few studies have reported the efficacy of RY gastric bypass to treat T2DM in patients with a BMI < 35 kg/m2 with remission rate from 48.1% to 100% [17,18,19,20]. In Korea, gastric cancer surgery is popular, so we experienced many postgastrectomy diabetic patients of low BMI. Two retrospective studies of gastric cancer surgery patients reported remission rates of 15.1% with mean 33.7-month follow-up, and 19.7% with one-year follow-up. Their preoperative BMI was 24.7 and 23.9 kg/m2, respectively [21,22]. Some studies of prospective duodenojejunal exclusion or RY bypass reconstruction to treat diabetes after gastrectomy in gastric disease (mainly cancer) patients with diabetes reported their results. In a study from Chile, the authors reported 65% remission with RY gastrojejunostomy or esophagojejunostomy in patients with a mean BMI of 29.1 kg/m2, while another study from China reported 38.1% with RY gastrojejunostomy in patients with a mean BMI of 23.8 kg/m2 [23,24]. We tried metabolic surgery to gastric cancer patients with T2DM with intestinal diversion after distal gastrectomy and node dissection for cancer. We modified conventional RY gastrojejunostomy by lengthening the biliopancreatic and alimentary limbs to make a similar structure as RY gastric bypass bariatric surgery to evaluate the clinical effects of surgery on diabetic control. All BMIs were < 35 kg/m2, the total length of bypassed jejunal limbs (the sum of Roux and Y limbs) exceeded 100 cm, but were shorter than or similar to those of RY gastric bypass. In our study, remission rate of DM was 30% and clinical improvement was observed in 20% of the enrolled patients. The remission rate was slightly lower than those of previously reported studies about RY gastric bypass. It is presumed that our study group included patients with a relatively shorter length of the total bypassed jejunum and alimentary limb (RY limb). In fact, in the previous studies on T2DM patients with BMI < 35 kg/m2, the total length of the bypassed jejunum was in the range of 200-250 cm and the length of the RY limb was 150 cm [17,18,19,20]. In contrast, 26 patients (87%) in the present study had a total bypassed jejunum length of <200 cm and 29 patients (96.7%) had an RY limb length of <150 cm. Although there was no statistical significance (P = 0.095), the total length of bypassed jejunum in effective group (mean length, 170.3 ± 29.7 cm) was longer than the ineffective group (mean length, 153.0 ± 40.2 cm). Statistical significance was observed in comparison of length of biliopancreatic limb between effective group and ineffective group (P = 0.049). It appears that the lack of significance was due to the small sample size and longer jejunal bypass could be more effective to achieve remission of DM. Moreover, the longer length of RY limbs is an important and fundamental component according to another hypothesis about the effectiveness after RY gastric bypass for DM control in that glucose homeostasis may stem from reprogramming of the intestinal glucose metabolism within the reconfigured RY limb to meet the increased bioenergetics demands of tissue growth and maintenance in response to its exposure to undigested nutrients [25]. To determine adequate length of bypassed jejunum for DM remission without malnutrition and other metabolic complication, it is necessary not only to accumulate a large number of cases with various length of bypassed jejunum but also to investigate nutritional status after surgery related to the length of common channel.
Although the exact mechanism of RY gastric bypass is not fully understood, previously reported studies propose that malabsorptive bariatric procedures such as RY gastric bypass or biliopancreatic diversion involving bypass of food might improve T2DM by increasing insulin sensitivity and improving beta cell function, as well as weight loss and reduced caloric intake [26]. In our study, there was a statistically significant difference in BMI reduction ratio between the effective and ineffective groups one year after surgery. Among six patients with exacerbation of DM at that time, the change of BMI between postoperative 6 months and postoperative 1 year was decreased in four, increased in one and stationary in one. We speculate that the reduction of BMI contributed to the improvement of DM but not to maintenance of diabetic improvement.
Two hypotheses have been proposed to explain T2DM remission after bariatric surgery with rearrangements of gastrointestinal tract exerting several discrete antidiabetic effects. The hindgut hypothesis states that surgical rerouting of nutrients to the distal part of the small bowel results in increased secretion and concomitant glucose lowering effects of glucagon like peptide-1 (GLP-1). The foregut hypothesis emphasizes that foregut or duodenojejunal bypass prevents release of unidentified nutrient induced diabetogenic signal [27]. In our analysis, greater BMI reduction, shorter duration of DM and lower preoperative HbA1c level was positive predictors of diabetic control. Among these, lower preoperative HbA1c level was most potent predictor on DM remission after surgery with marginal significance (P = 0.0495). In previous study, patients having shorter duration and less severe DM have higher rates of remission after surgery and the magnitude of weight loss appears to increase the likelihood of remission in obese individuals with type 2 DM [28,29,30]. Although, these studies involved T2DM patients with high BMI (>35 kg/m2), results of the present study correspond with the results of earlier studies that reported that in patients with shorter duration and lesser severe DM, DM can be alleviated by bariatric surgery such as RY gastric bypass. Thus, we postulate that early surgical intervention might be beneficial for remission of T2DM. Huang et al. [18] reported that in patients with BMI < 35 kg/m2, the resolution of type 2 DM was not related to changes in BMI, although a higher preoparative BMI did predict remission. Our results about effects of BMI changes and preoperative BMI value differ.
Limitations of the study include the small case number and the relatively short-term follow-up (12 months). It is possible that analysis has limited statistical significance due to small sample size and some of patients with remission of diabetes after surgery relapsed over time. Another limitation is the lack of preoperative and postoperative gut hormone data, as well as insulin and C-peptide levels. Without data on accompanying change in gut hormone, insulin and C-peptide, we cannot demonstrate the mechanisms for the remission of T2DM after surgery. Another limitation is that cancer recurrence and adjuvant chemotherapy for advanced gastric cancer could affect glucose level. The most common side effect of chemotherapy is anorexia with nausea and vomiting and these symptoms can also result from a malignant state, however, in our study, there was no cancer recurrence in regular follow-up examination and adjuvant chemotherapy was already finished at one year follow-up (7 patients).
Future studies will focus on the long-term follow-up and case accumulation with a variety of lengths of bypassed jejunum longer than those of the present study in order to support surgery as a treatment option in nonmorbid obese patients T2DM. To remove bias due to adjuvant chemotherapy and advanced cancer state, it is necessary to choose early stage cancer as study subjects and to investigate a dietary amount and habitus of patients. Furthermore, studies of gut hormone, insulin, C-peptide and postoperative nutritional parameter are needed to clarify the mechanism and determine the appropriate length of bypassed jejunum.
In conclusion, the present study indicates that long RY gastrojejunostomy may play a role in remission or improvement of T2DM patients with BMI < 35 kg/m2. Greater BMI reduction, shorter duration of T2DM and lower preoperative HbA1c level are possible positive predictors. Gastrectomy and modified gastrointestinal reconstruction in gastric cancer surgery is a useful clinical research model of metabolic surgery T2DM, especially of low BMI patients.
Figures and Tables
Fig. 1
Diagram of long Roux-en-Y gastrojejunostomy. The length of alimentary limb (A, from the gastrojejunostomy site to the jejunojejunostomy site): 60-150 cm. The length of biliopancreatic limb (B, from the ligament of Treitz to the jejunojejunostomy site): 30-120 cm.
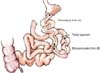
Table 3
Univariable analysis at postoperative 1 year

Values are presented as mean ± standard deviation.
Effective group: remission group + improvement group; Ineffective group: stationary group. Preop, preoparative; HbA1c, glycated hemoglobin; FBS, fasting blood glucose; BMI, body mass index; DM, diabetes mellitus.
a)BMI reduction ratio (%) = (postoperative BMI/preoperative BMI) × 100.
References
1. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012; 366:1577–1585.
2. Shukla AP, Ahn SM, Patel RT, Rosenbaum MW, Rubino F. Surgical treatment of type 2 diabetes: the surgeon perspective. Endocrine. 2011; 40:151–161.
3. Dixon JB, Zimmet P, Alberti KG, Rubino F. International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Arq Bras Endocrinol Metabol. 2011; 55:367–382.
4. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004; 292:1724–1737.
5. Chiellini C, Rubino F, Castagneto M, Nanni G, Mingrone G. The effect of biliopancreatic diversion on type 2 diabetes i n pat ients wit h BMI <35 kg/m2. Diabetologia. 2009; 52:1027–1030.
6. Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Lee YC. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg. 2012; 16:45–51.
7. Shimizu H, Timratana P, Schauer PR, Rogula T. Review of metabolic surgery for type 2 diabetes in patients with a BMI <35kg/m(2). J Obes. 2012; 2012:147256.
8. Lanzarini E, Csendes A, Gutierrez L, Cuevas P, Lembach H, Molina JC, et al. Type 2 diabetes mellitus in patients with mild obesity: preliminary results of surgical treatment. Obes Surg. 2013; 23:234–240.
9. DePaul a, Macedo AL, Rassi N, Machado CA, Schraibman V, Silva LQ, et al. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc. 2008; 22:706–716.
10. Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, et al. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010; 147:664–669.
11. Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011; 146:143–148.
12. Rubino F, Marescaux J. Effect of duodenaljejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004; 239:1–11.
13. Cohen RV, Schiavon CA, Pinheiro JS, Correa JL, Rubino F. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22-34 kg/m2: a report of 2 cases. Surg Obes Relat Dis. 2007; 3:195–197.
14. Ramos AC, Galvao Neto MP, de Souza YM, Galvao M, Murakami AH, Silva AC, et al. Laparoscopic duodenal-jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI<30 kg/m2 (LBMI). Obes Surg. 2009; 19:307–312.
15. Ferzli GS, Dominique E, Ciaglia M, Bluth MH, Gonzalez A, Fingerhut A. Clinical improvement after duodenojejunal bypass for nonobese type 2 diabetes despite minimal improvement in glycemic homeostasis. World J Surg. 2009; 33:972–979.
16. Geloneze B, Geloneze SR, Fiori C, Stabe C, Tambascia MA, Chaim EA, et al. Surgery for nonobese type 2 diabetic patients: an interventional study with duodenaljejunal exclusion. Obes Surg. 2009; 19:1077–1083.
17. Boza C, Munoz R, Salinas J, Gamboa C, Klaassen J, Escalona A, et al. Safety and efficacy of Roux-en-Y gastric bypass to treat type 2 diabetes mellitus in nonseverely obese patients. Obes Surg. 2011; 21:1330–1336.
18. Huang CK, Shabbir A, Lo CH, Tai CM, Chen YS, Houng JY. Laparoscopic Rouxen-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25-35. Obes Surg. 2011; 21:1344–1349.
19. Kim WS, Kim JW, Ahn CW, Choi SH. Resolution of type 2 diabetes after gastrectomy for gastric cancer with long limb Roux-en Y reconstruction: a prospective pilot study. J Korean Surg Soc. 2013; 84:88–93.
20. Shah SS, Todkar JS, Shah PS, Cummings DE. Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index <35 kg/m(2). Surg Obes Relat Dis. 2010; 6:332–338.
21. Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012; 18:49–54.
22. Lee W, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, et al. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obes Surg. 2012; 22:1238–1243.
23. Lanzarini E, Csendes A, Lembach H, Molina J, Gutierrez L, Silva J. Evolution of type 2 diabetes mellitus in non morbid obese gastrectomized patients with Roux en-Y reconstruction: retrospective study. World J Surg. 2010; 34:2098–2102.
24. Yang J, Li C, Liu H, Gu H, Chen P, Liu B. Effects of subtotal gastrectomy and Rouxen-Y gastrojejunostomy on the clinical outcome of type 2 diabetes mellitus. J Surg Res. 2010; 164:e67–e71.
25. Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013; 341:406–410.
26. Mari A, Manco M, Guidone C, Nanni G, Castagneto M, Mingrone G, et al. Restoration of normal glucose tolerance in severely obese patients after biliopancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006; 49:2136–2143.
27. Knop FK. Resolution of type 2 diabetes fol low i ng g ast r ic by pass surger y: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia. 2009; 52:2270–2276.
28. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003; 238:467–484.
29. Hall TC, Pellen MG, Sedman PC, Jain PK. Preoperative factors predicting remission of type 2 diabetes mellitus after Rouxen-Y gastric bypass surgery for obesity. Obes Surg. 2010; 20:1245–1250.
30. Torquati A, Lutfi R, Abumrad N, Richards WO. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg. 2005; 9:1112–1116.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download