Abstract
Purpose
The present study aimed to evaluate the risk factors and the role of graft material in the development of an acute phase systemic inflammatory response, and the clinical outcome in patients who undergo endovascular aneurysm repair (EVAR) or open surgical repair (OSR) of an abdominal aortic aneurysm (AAA).
Methods
We retrospectively evaluated the risk factors and the role of graft material in an increased risk of developing systemic inflammatory response syndrome (SIRS), and the clinical outcome in patients who underwent EVAR or OSR of an AAA.
Results
A total of 308 consecutive patients who underwent AAA repair were included; 178 received EVAR and 130 received OSR. There was no significant difference in the incidence of SIRS between EVAR patients and OSR patients. Regardless of treatment modality, SIRS was observed more frequently in patients treated with woven polyester grafts. Postoperative hospitalization was significantly prolonged in patients that experienced SIRS. In multivariate analyses, the initial white blood cell count (P = 0.001) and the use of woven polyester grafts (P = 0.005) were significantly associated with an increased risk of developing SIRS in patients who underwent EVAR. By contrast, the use of woven polyester grafts was the only factor associated with an increased risk of developing SIRS in patients who underwent OSR, although this was not statistically significant (P = 0.052).
In patients who undergo endovascular aneurysm repair (EVAR) for an abdominal aortic aneurysm (AAA), an acute phase systemic inflammatory response, known as postimplantation syndrome (PIS) and defined as continuous pyrexia coinciding with a rise in inflammatory markers despite antibiotic therapy, may occur shortly after implantation [1,2,3,4]. Although PIS is thought to be transient and harmless in most cases, its impact on the outcome of patients is a concern because it may lead to prolonged hospitalization and a more complicated postoperative recovery [3,4]. Since it was first described by Velazquez et al. [5] in 1999, many laboratory and clinical data have suggested that the development of systemic inflammatory response syndrome (SIRS) following EVAR is due to activation of the inflammatory cascade [6].
The incidence of PIS in AAA patients has been reported to vary widely between 14% and 60% [1]. Several studies have compared the biological responses between EVAR and open surgical repair (ORS) for treatment of an AAA; however, there is no consensus regarding whether the inflammatory response is greater during EVAR or during OSR [2,7,8,9]. In patients who undergo EVAR, this response is primarily attributed to endothelial dysfunction reflecting a synergic role of the graft material and the endovascular surgical technique. Several studies have concluded that the composition of stent grafts may affect the incidence of PIS [1,2,3,4].
Despite its benign nature in most patients, the clinical significance of PIS is unknown and there are no clear guidelines for its management [4]. For optimal management of patients with PIS, a better understanding of the cause of the inflammatory response is necessary. The aim of this study was to evaluate the risk factors and the role of graft material in the increased risk of developing an acute phase systemic inflammatory response, and the clinical outcome in patients who undergo EVAR or OSR of an AAA.
This was a retrospective, observational study using data extracted from medical records. The study protocol was approved by Asan Medical Center Institutional Review Board. A total of 423 consecutive patients who underwent an AAA repair at our institution from January 2008 to December 2012 were included in the study. Of these patients, 206 (48.7%) received EVAR and 217 (51.3%) received OSR. The treatment modality used to repair an AAA, namely, EVAR or OSR, was determined by the morphology of the AAA based on the preoperative CT angiogram, the patient's comorbidities, and a thorough explanation of the risks and benefits of each treatment modality.
Exclusion criteria for the present study included ruptured AAA, clinical and/or laboratory evidence of a recent infection, previous major trauma or surgery 2 weeks prior to enrollment, combined major operation, preoperative signs of limb ischemia, and postoperative complications related to AAA repair. Of the 206 patients who underwent EVAR, 28 patients (13.6%) were excluded from the study for the following reasons: ruptured AAA (n = 2), evidence of a recent infection (n = 10; 5 cases of foot ulcer, 2 upper respiratory infection, 2 cholecystitis, and 1 cholangitis), previous trauma or surgery (n = 3), combined major operation (n = 1), and postoperative complications (n = 12; 3 cases of pneumonia, 3 wound infection, 2 other arterial dissection, 1 cardiovascular accident, 1 distal embolization, 1 limb graft occlusion, and 1 ischemic colitis). Endovascular procedures were performed under general or regional anesthesia and followed a standard vascular protocol. Of the 217 patients who underwent OSR, 87 patients (40.1%) were excluded from the study for the following reasons: ruptured AAA (n = 33), evidence of a recent infection (n = 16; 5 cases of upper respiratory tract infection, 4 foot ulcer, 4 infected AAA, 1 cholecystitis, 1 urinary tract infection, and 1 esophagitis), combined major operation (n = 4), preoperative signs of limb ischemia (n = 9), and postoperative complications (n = 25; 7 cases of pneumonia, 6 wound infection, 4 cardiovascular accident, 2 distal embolization, 1 acute renal failure, 1 bladder rupture, 1 Herpes esophagitis, 1 bleeding, 1 pseudomembranous colitis, and 1 cholecystitis). All open surgical procedures were performed under general anesthesia and followed a standard technique of transperitoneal approach. According to the hospital protocol, all patients included in this study received prophylactic antibiotics 30 minutes before the operation as well as 5,000 units of heparin intravenously before introduction of the stent graft deployment system during EVAR or before aortic cross-clamping during OSR.
Demographics, body mass index, risk factors of interest, and other data, including clinical presentation, morphologic characteristics of the aneurysm, operative and postoperative characteristics, periprocedural blood loss, hospitalization, and patient survival, were recorded for each patient. Temperature was recorded every 8 hours during the entire duration of hospitalization. Body temperature, WBC and platelet counts, and serum CRP concentration were serially assessed 1 day before AAA repair and during hospitalization, depending on the clinical status of the patient. The highest pre- and postoperative values and the changes in the WBC count, platelet count, and CRP concentration were considered for the analysis. Patients were discharged in the absence of any complications, as confirmed by the follow-up CT angiogram, with a body temperature < 37.5℃ for at least 24 hours and a WBC count < 12,000/mm3. All data were collected prospectively for all consecutive patients in an Excel database (Microsoft Co., Redmond, WA, USA) and analyzed retrospectively.
PIS was defined as continuous temperature > 38℃ and a WBC count > 12,000/mm3 despite antibiotic therapy and negative culture results. This definition is in accordance with the definition of SIRS [10,11], with PIS fulfilling at least two of the four SIRS criteria. Four SIRS criteria are: (1) body temperature < 36℃ or > 38℃, (2) heart rate > 90 beats/min, (3) tachypnea > 20 breaths/min or an arterial partial pressure of carbon dioxide < 32 mmHg, and (4) a WBC count < 4,000/mm3 or > 12,000/mm3, or the presence of greater than 10% immature neutrophils (band forms) [1]. In the present study, we used the definition of SIRS in patients presenting with an acute systemic inflammatory response after an elective repair of AAA. To evaluate the role of the graft component used for EVAR or OSR in an increased risk of developing SIRS, enrolled patients were divided into two groups, namely, SIRS and non-SIRS, and the graft composition was divided into woven polyester and expanded polytetrafluoroethylene (PTFE).
Categorical variables presented as counts and percentages were analyzed using the chi-square test and Fisher exact test, as appropriate. Continuous variables presented as mean ± standard deviation were compared using the Student t-test. A logistic regression analysis was used to evaluate risk factors for SIRS following AAA repair. Patients who underwent EVAR and OSR were analyzed separately to identify statistically significant variables in each treatment modality. The variables that showed significance with a P-value cutoff of 0.1 in univariate analysis were introduced into a multivariate logistic regression model. Multivariate analysis using a backward elimination was performed to determine independent significance. All other statistical analyses were carried out using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA), with a P-value of ≤0.05 considered statistically significant.
Of 423 patients who underwent elective repair of an AAA, 308 consecutive patients were included in the present study, in accordance with our inclusion criteria; 178 (57.8%) received EVAR and 130 (42.2%) received OSR (Fig. 1). The diagnosis of SIRS was established in 110 patients (35.7%) who underwent an elective AAA repair: 57 EVAR patients (32.0%) and 53 OSR patients (40.8%). There was no significant difference in the incidence of SIRS between the patients who underwent EVAR and those who underwent OSR (P = 0.113).
The demographics and clinical characteristics of the patients are presented in Tables 1, 2. In both EVAR and OSR patients, no statistically significant differences were observed in demographics, atherosclerotic risk factors, clinical characteristics, or the morphology of the AAA between the two groups (SIRS group and non-SIRS group); the exception was the incidence of a symptomatic aneurysm in EVAR patients (P = 0.002). In patients who underwent EVAR, administration of statins was significantly more common in the non-SIRS group than in the SIRS group (P = 0.079), while there was no significant difference between the groups in patients who underwent OSR (P = 0.185).
According to the graft composition, the grafts used for AAA repair were divided into woven polyester (EVAR, n = 140; OSR, n = 96) and expanded PTFE (EVAR, n = 38; OSR, n = 34). The incidence of SIRS was significantly higher in patients who received woven polyester grafts (EVAR, 37.9%; OSR, 45.8%) than in patients who received expanded PTFE grafts (EVAR, 10.5%; OSR, 26.5%) in both EVAR (P = 0.001) and OSR (P = 0.048) patients.
Body temperature and pre- and postoperative laboratory data are shown in Tables 3, 4. In both EVAR and OSR patients, there were no significant differences in mean baseline body temperature or the preoperative CRP concentration or platelet count between the two groups. By contrast, the preoperative WBC count was significantly higher in the SIRS group than in the non-SIRS group (EVAR, P = 0.001; OSR, P = 0.020), although all values were within the normal range. The incidence of postoperative fever (body temperature > 38℃) in EVAR patients was significantly higher in the SIRS group than in the non-SIRS group (P = 0.001), but there was no significant difference between the two groups in OSR patients (P = 0.394). The onset and duration of fever did not significantly differ between the two groups. With regard to postoperative laboratory data, the highest mean WBC count was significantly higher in the SIRS group than in the non-SIRS group in both EVAR patients (P = 0.001) and OSR patients (P = 0.001). However, there was no significant difference in the lowest mean platelet count between the two groups (EVAR, P = 0.891; OSR, P = 0.387). The highest mean CRP concentration was significantly higher in the SIRS group than in the non-SIRS group in EVAR patients (P = 0.001), whereas there was no significant difference between the groups in OSR patients (P = 0.239). With regard to the postoperative increase in the WBC count compared to the preoperative baseline value, this increase was significantly larger in the SIRS group than in the non-SIRS group (EVAR, P = 0.001; OSR, P = 0.047). In patients who underwent EVAR, there was a clinically significant difference in the postoperative decrease in the platelet count between the two groups, but this was not statistically significant (P = 0.057), whereas no significant difference was noted in patients who underwent OSR (P = 0.387). The postoperative increase in CRP concentration was significantly larger in the SIRS group than in the non-SIRS group in patients who underwent EVAR (P = 0.001), while there was no significant difference between the two groups in patients who underwent OSR (P = 0.262). In all patients in the SIRS group, blood, urine, and sputum cultures were negative. Postoperative clinical outcomes showed no significant differences between the two groups. Although postoperative hospitalization was significantly longer in the SIRS group than in the non-SIRS group (EVAR, P = 0.022; OSR, P = 0.021) (Tables 1, 2), all patients were discharged from hospital after uncomplicated recoveries.
In multivariate analyses of risk factors for SIRS stratified by treatment modality, the preoperative WBC count (hazard ratio [HR], 3.24; 95% confidence interval [CI], 1.64-6.40; P = 0.001) and the use of woven polyester grafts (HR, 4.89; 95% CI, 1.61-14.86; P = 0.005) were significantly associated with an increased risk of developing SIRS in patients who underwent EVAR. By contrast, the use of woven polyester grafts (HR, 2.35; 95% CI, 0.99-5.56; P = 0.052) was the only factor associated with an increased risk of developing SIRS in patients who underwent OSR, but this was not statistically significant.
An acute phase systemic inflammatory response, known as PIS, affects a significant number of patients who undergo EVAR for the management of an AAA, and the incidence of this response varies widely between 14% and 60% [1,2,3,4]. However, the mechanisms behind this systemic inflammatory response are not well understood, and its clinical significance is unclear [1,2,3,4,5,6,7]. Furthermore, there is no consensus about the definition of this phenomenon [1,5,12,13]; a widely accepted definition of PIS is needed to evaluate and compare variables that are reportedly related to this syndrome [1]. In our opinion, a definition according to SIRS seems to be reasonable because there is evidence that a systemic inflammatory response is induced by implantation of an endovascular graft [1,14,15]. PIS fulfills at least two of the SIRS criteria [1,10,11] and thus may represent a state of SIRS itself. The present study included 308 consecutive patients in accordance with our inclusion criteria, and definition of SIRS was used to determine whether these patients had an acute phase systemic inflammatory response.
Since this systemic inflammatory response was first described by Velazquez et al. [5] in 1999, many laboratory and clinical data have suggested that it represents an inflammatory cascade that is activated by complex immunological changes following EVAR, leading to the development of SIRS [6,16,17,18]. Although the trigger for this immune response is unclear, differences between the types of aortic grafts used and the development of a systemic inflammatory response indicate that the graft composition can influence the inflammatory response [1]. Both EVAR and OSR can provoke an inflammatory response [9]. This response appears to be different from the more generalized inflammatory response seen after OSR of an AAA, which is due to extensive surgical trauma and reperfusion injury [16,17,18]. In one study, this inflammatory response was greater during OSR than during EVAR [9]; however, there is no consensus regarding whether the inflammatory response is greater during EVAR or during OSR [2,8,9]. In the current study, which included all patients who underwent EVAR or OSR, the incidence of SIRS did not differ between OSR patients and EVAR patients. However, the inflammatory response may have been greater during EVAR than during OSR, according to the laboratory data. Regardless of the treatment modality, SIRS was observed more frequently in patients that received woven polyester grafts than in those that received expanded PTFE grafts. The administration of statins before surgery attenuates perioperative inflammation [19,20,21]. In this study, statin use showed a tendency of decrease in the development of SIRS in patients who underwent EVAR (P = 0.079), while there was no significant preventative effect in patients who underwent OSR (P = 0.185). This difference may be due to the less extensive surgical trauma and reperfusion injury following EVAR than following OSR, in combination with the limitation of the inflammatory response by statins. By contrast, the demographics and risk factors of the patients, the maximum diameter of the aneurysm, the operating time, and the amount of blood transfused during the operation did not correlate with SIRS following EVAR or OSR. However, in addition to its retrospective, nonrandomized nature, the fact that we did not assess inflammatory markers is a limitation of this study. Further prospective investigations, with a larger number of patients and assessment of inflammatory markers over a longer period, are necessary to draw definite conclusions about the impact of the graft composition on the increased risk of developing SIRS and to develop clear guidelines for its management.
An acute phase systemic inflammatory response following EVAR is thought to be transient and harmless in most cases, and, in this study, there was no EVAR-related endovascular graft infection during the study period. However, despite its benign nature, the impact of this response on the clinical outcome of patients is a concern because it may lead to prolonged hospitalization and a more complicated postoperative recovery [3,4]. Regardless of the treatment modality, our data showed that SIRS was, indeed, associated with prolonged hospitalization (EVAR, P = 0.022; OSR, P = 0.021) and thus this might affect the quality of the patient's life and the cost-effectiveness of the elective repair of AAAs. Although none of the patients in this study had any perioperative major complications, several studies reported that a serious inflammatory response might result in an extensive endothelial injury, owing to the direct effects of the mediating agents and the WBC-endothelial interaction. This can lead to an increased risk of postoperative morbidity, such as pulmonary dysfunction, cardiovascular events, renal insufficiency, and multisystem organ failure, particularly in high-risk patients [6,22,23,24]. In the future, studies of larger cohorts are warranted to identify risk factors for the development of a severe systemic inflammatory response, as well as to identify strategies that can control the inflammatory process after the elective repair of AAAs.
Our study has several limitations. First, it was performed retrospectively, and the decision of whether EVAR or OSR was performed was mainly made by the surgeon based on the expected level of technical difficulty of the procedure. Second, we did not assess other important inflammatory markers. Finally, the number of patients that received expanded PTFE grafts was relatively small. Hence, the incidence of an acute systemic inflammatory response may have been underestimated in these patients. In the future, studies on larger cohorts are warranted.
In summary, SIRS is a relatively common complication of a benign nature after the elective repair of AAAs, but it leads to prolonged hospitalization of both EVAR and OSR patients. Although the incidence of SIRS does not significantly differ between patients that undergo EVAR and those that undergo OSR, laboratory data indicate that the inflammatory response is greater during EVAR than during OSR. The current study shows that an increased risk of developing SIRS is significantly associated with the preoperative WBC count and the use of woven polyester grafts in patients that undergo EVAR. By contrast, the use of woven polyester grafts is the only factor associated with an increased risk of developing SIRS in patients that undergo OSR, although this was borderline significant.
Figures and Tables
Fig. 1
Flow chart of patient inclusion. AAA, abdominal aortic aneurysm; EVAR, endovascular aneurysm repair; OSR, open surgical repair; SIRS, systemic inflammatory response syndrome.
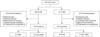
Table 1
Perioperative patient characteristics according to the presence or absence of SIRS in patients who underwent endovascular aneurysm repair

Table 2
Perioperative patient characteristics presented according to the presence or absence of SIRS in patients who underwent open surgical repair

Table 3
Body temperature and pre- and postoperative laboratory data of the patients who underwent endovascular aneurysm repair

References
1. Arnaoutoglou E, Kouvelos G, Milionis H, Mavridis A, Kolaitis N, Papa N, et al. Post-implantation syndrome following endovascular abdominal aortic aneurysm repair: preliminary data. Interact Cardiovasc Thorac Surg. 2011; 12:609–614.
2. Gerasimidis T, Sfyroeras G, Trellopoulos G, Skoura L, Papazoglou K, Konstantinidis K, et al. Impact of endograft material on the inflammatory response after elective endovascular abdominal aortic aneurysm repair. Angiology. 2005; 56:743–753.
3. Moulakakis KG, Alepaki M, Sfyroeras GS, Antonopoulos CN, Giannakopoulos TG, Kakisis J, et al. The impact of endograft type on inflammatory response after endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 2013; 57:668–677.
4. Voute MT, Bastos Gonçalves FM, van de Luijtgaarden KM, Klein Nulent CG, Hoeks SE, Stolker RJ, et al. Stent graft composition plays a material role in the postimplantation syndrome. J Vasc Surg. 2012; 56:1503–1509.
5. Velazquez OC, Carpenter JP, Baum RA, Barker CF, Golden M, Criado F, et al. Perigraft air, fever, and leukocytosis after endovascular repair of abdominal aortic aneurysms. Am J Surg. 1999; 178:185–189.
6. Arnaoutoglou E, Papas N, Milionis H, Kouvelos G, Koulouras V, Matsagkas MI. Post-implantation syndrome after endovascular repair of aortic aneurysms: need for postdischarge surveillance. Interact Cardiovasc Thorac Surg. 2010; 11:449–454.
7. Park HS, Jung IM, Soh YH, Cho BS, Ahn YJ, Chung JK. Experience of non-vascular complications following endovascular aneurysm repair for abdominal aortic aneurysm. J Korean Surg Soc. 2011; 80:Suppl 1. S67–S70.
8. Odegard A, Lundbom J, Myhre HO, Hatlinghus S, Bergh K, Waage A, et al. The inflammatory response following treatment of abdominal aortic aneurysms: a comparison between open surgery and endovascular repair. Eur J Vasc Endovasc Surg. 2000; 19:536–544.
9. Swartbol P, Truedsson L, Norgren L. The inflammatory response and its consequence for the clinical outcome following aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2001; 21:393–400.
10. Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992; 101:1481–1483.
11. Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997; 25:1789–1795.
12. Gorich J, Rilinger N, Soldner J, Kramer S, Orend KH, Schutz A, et al. Endovascular repair of aortic aneurysms: treatment of complications. J Endovasc Surg. 1999; 6:136–146.
13. Blum U, Voshage G, Lammer J, Beyersdorf F, Tollner D, Kretschmer G, et al. Endoluminal stent-grafts for infrarenal abdominal aortic aneurysms. N Engl J Med. 1997; 336:13–20.
14. Galle C, De Maertelaer V, Motte S, Zhou L, Stordeur P, Delville JP, et al. Early inflammatory response after elective abdominal aortic aneurysm repair: a comparison between endovascular procedure and conventional surgery. J Vasc Surg. 2000; 32:234–246.
15. Morikage N, Esato K, Zenpo N, Fujioka K, Takenaka H. Is endovascular treatment of abdominal aortic aneurysms less invasive regarding the biological responses? Surg Today. 2000; 30:142–146.
16. Akowuah E, Wilde P, Angelini G, Bryan AJ. Systemic inflammatory response after endoluminal stenting of the descending thoracic aorta. Interact Cardiovasc Thorac Surg. 2007; 6:741–743.
17. Norgren L, Swartbol P. Biological responses to endovascular treatment of abdominal aortic aneurysms. J Endovasc Surg. 1997; 4:169–173.
18. Swartbol P, Norgren L, Albrechtsson U, Cwikiel W, Jahr J, Jonung T, et al. Biological responses differ considerably between endovascular and conventional aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 1996; 12:18–25.
19. Voute MT, Winkel TA, Poldermans D. Optimal medical management around the time of surgery. Heart. 2010; 96:1842–1848.
20. Voute MT, Winkel TA, Poldermans D. Safety of fluvastatin in patients undergoing high-risk non-cardiac surgery. Expert Opin Drug Saf. 2010; 9:793–800.
21. Schouten O, Boersma E, Hoeks SE, Benner R, van Urk H, van Sambeek MR, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009; 361:980–989.
22. Chang CK, Chuter TA, Niemann CU, Shlipak MG, Cohen MJ, Reilly LM, et al. Systemic inflammation, coagulopathy, and acute renal insufficiency following endovascular thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2009; 49:1140–1146.
23. Gabriel EA, Locali RF, Matsoka PK, Romano CC, Duarte AJ, Buffolo E. First inflammatory risk score for aortic endoprostheses. Rev Bras Cir Cardiovasc. 2008; 23:512–518.
24. Cross KS, Bouchier-Hayes D, Leahy AL. Consumptive coagulopathy following endovascular stent repair of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2000; 19:94–95.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download