Abstract
Purpose
Surgical excision is the definitive treatment for localized recurrence of papillary thyroid carcinoma. Reoperation for recurrence, however, is challenging and associated with increased operative times and complication rates. For safe and effective reoperation, ultrasound-guided charcoal tattooing localization can be used. The aim of this study was to investigate the feasibility and safety of the ultrasound-guided charcoal tattooing localization.
Methods
Between November 2012 and August 2013, ten patients underwent preoperative charcoal tattooing localization for twelve recurrent lesions. Patient demographics, pathologic features, and operation results were reviewed.
Results
The technical success rate of charcoal tattooing was 100%. Eight patients had one recurrent lesion, and two patients had double lesions. Among these 12 recurrent lesions, three (25%) were found in level II, four (33%) in level IV, four (33%) in level VI, and one (8%) was found in the thyroidectomy bed site. The mean size of lesions was 0.87 ± 0.35 cm. Of these 10 patients, eight patients underwent selective lymph node dissection, one patient underwent modified radical neck dissection, and one patient underwent recurrent mass excision. Transient hypocalcemia developed in one patient, and no recurrent laryngeal nerve palsy occurred. There were no major complications related to the injection of the charcoal. The mean follow-up period after reoperation was 8.6 ± 2.7 months; in the follow-up ultrasound, there were no remnant lesions in all patients.
Thyroid cancer is the most prevalent cancer in Korea, and its incidence has increased strikingly [1]. The age-adjusted incidence rates per 100,000 persons increased from 2.1 and 10.4 in 1999 to 15.4 and 79.6 in 2009 in men and women, respectively [2]. Cho et al. [1] reported that the cumulative recurrence rates of thyroid cancer were 18% at 10 years and 31% at 20 years in Korea. The high sensitivity of ultrasound (US) and thyroglobulin measurement allowed early detection of smaller nonpalpable recurrences [3]. Although repeated radioiodine treatment alone may effectively eliminated small recurrent tumors, up to 30% of these recurrent tumors will show no radioiodine uptake; a more aggressive surgical approach may be warranted [4].
Reoperation for recurrent thyroid cancer is challenging and associated with increased operative times and complication rates when compared with initial surgery [5,6]. For safe and effective reoperation, several techniques for the localization of recurrent lesions have been reported [7,8,9,10,11,12]. US-guided charcoal tattooing localization has been used for more than 15 years for tattooing breast cancer and this technique is easy and innocuous to implement [13]. There is some controversy, however, as to whether charcoal tattooing is feasible and safe for patient with recurrent thyroid carcinoma.
The aim of this study was to investigate the feasibility and safety of the US-guided charcoal tattooing localization.
The Institutional Review Board of Seoul National University Hospital approved this study (H-1401-002-543), and the need for written informed consent was waived. Ten patients with papillary thyroid carcinoma underwent charcoal tattooing localization for 12 recurrent regions between November 2012 and August 2013. All patients were diagnosed with recurrent thyroid cancer by fine-needle aspiration cytology and/or washout thyroglobulin of the suspected lesions.
For US-guided charcoal tattooing, we used an US machine (HD3; Philips Healthcare, Ridgedfield Park, NJ, USA) with a high-frequency transducer (7-12 MHz), 4% Activated Charcoal, and a 1-mL syringe with a 22-gauge needle. One day before or at the operation day, tattooing was performed by the radiologists without local anesthesia.
In brief, the patient was placed in a supine position with neck extension. After preparing the skin with an alcohol wipe, a 1-mL syringe fitted with a 22-gauge needle was inserted into the tumor under US guidance. Once the needle tip was located inside the tumor, 4% activated charcoal was injected slowly (Fig. 1). The amount of charcoal was adjusted according to the tumor size, from 0.2 to 1.5 mL. After tattooing, the injection site was compressed gently for 3 minutes to prevent hematoma.
Vocal cords were examined using video-assisted or direct laryngoscopy 1 day before surgery. Serum levels of calcium, phosphorus, ionized calcium, and parathyroid hormone were measured on postoperative Day 1. All patients were followed up at 2 weeks, 3 months, 6 months, and annually. Follow-up tests included clinical examinations for hypoparathyroidism, vocal cord evaluation, thyroid function test, and surveillance US. In cases where vocal cord injury was noted in the previous follow-up, the vocal cord was re-examined using video-assisted or direct laryngoscopy. In cases where hypoparathyroidism was noted in the previous examination, serum levels of calcium, phosphorus, and parathyroid hormone were also measured.
The characteristics of the patients were summarized in Table 1. The mean age of the patients was 53.3 ± 14.9 years (range, 32-73 years), and number of neck operations before charcoal tattooing was 1.6 ± 1.07 (range, 1-4). Four patients underwent total thyroidectomy with central lymph node dissection as initial operation. Total thyroidectomy with modified lateral neck dissection was performed in six patients for lateral neck lymph node metastasis. Three patients were classified as TNM stage I, and seven were as stage IVA.
After initial operation, nine patients received radioactive iodine (RAI) ablation; two patients received single dose of RAI and seven patients underwent 2 to 5 times RAI ablation. The mean times of RAI ablation before reoperation was 2.2 (range, 0-5), and dose of 131I was 172 mCi (range, 60-290 mCi).
Time interval between prior surgery and reoperation was 48 ± 37.85 months (range, 10-128 months). Eight patients had one recurrent lesion, and two patients had double lesions (Table 2). Among these 12 recurrent lesions, three (25%) were found in level II, four (33%) in level IV, four (33%) in level VI, and one (8%) was found in the thyroidectomy bed site. The mean size of lesions was 0.87 ± 0.35 cm (range, 0.49-1.61 cm). All foci of recurrent lesions arose in the previously operated compartments. Of these 10 patients, eight patients underwent selective lymph node dissection, one patient underwent modified radical neck dissection, and one patient underwent recurrent mass excision. The operation time was 97.6 ± 27.9 minutes (range, 62-140 minutes). In pathologic examination, one ectopic thyroid, and one thyroidectomy bed site recurrence, and 10 lymph node recurrences were confirmed.
All patients had no change of vocal cord function after reoperation. Transient hypocalcemia developed in one patient and resolved after 2 weeks. No patient had esophageal or tracheal injury. There were no major complications related to the injection of the charcoal including hematomas and allergic reactions, while 1 patient had a dot-like marking at the skin puncture site after charcoal injection. The mean follow-up period after reoperation was 8.6 ± 2.7 months (range, 5-14 months). In the follow-up US, there were no remnant lesions in all patients.
Surgical excision is the most definitive treatment for localized recurrence. However, due to fibrosis and scar tissue formation in the surgical field, reoperation is technically challenging [12]. Moreover, reoperation can be associated with higher complication rates and increased operation time. The major complications of reoperation included recurrent laryngeal nerve (RLN) injury, hypoparathyroidism, and injury to major neural structures, such as lower division of the facial nerve, the accessory nerve, or the sympathetic trunk [14]. In cases of reoperation, permanent recurrent RLN occurred in 3%-15% and permanent hypoparathyroidism in 5% or more; whereas permanent RLN injury developed in 0%-3% and permanent hypoparathyroidism in 1%-2% of the patients who underwent a first thyroid operation. Furthermore, a recurrent lesion can be missed at the time of operation because of scarring, fibrosis, bleeding, and proximity of the tumor to the recurrent laryngeal nerve [15].
To perform the reoperation safely and effectively, several techniques including intraoperative US, preoperative US-guided skin marking, and radio-guided surgery using intraoperative gamma probe have been reported for the localization of recurrent lesions [7,8,9,10,11,12]. Although these techniques helped physicians to localize the recurrence, potential disadvantages also existed. Intraoperative US required the presence of an US machine in the operating room and a radiologist or a surgeon practicing US [7]. In preoperative US-guided skin marking, the skin marking no longer provides a reference point to mark the exact location of the recurrent lesion after subplatysmal flaps are elevated [9]. US-guided hook-wire localization was reported to have minimal complications from the needle placement; however, the needle could become dislocated during retraction. The hook needle could break during insertion, fall out, or migrate to an unexpected site [16]. Rubello et al. [17] reported the use of an intraoperative gamma probe after 131I administration showed a technical success rate of 80.6%. However, radio-guided surgery using 131I, 99mTc-sestamibi, and 18F-fuorodeoxiglucose required preoperative injection of material intravenously, and additional probes for detection of the targeted lesion [11,17,18]. Furthermore, false positive lesions including submandibular salivary glands and thymus, and false negative cases might arise when the lesion exhibits low iodine uptake [17]. Radio-guided surgery technique also exposes the hands of the surgeon to radiation, although the overall received dose was found to be minimal [19].
In this context, we chose and investigated charcoal tattooing localization method for patients with recurrent thyroid cancer. US-guided charcoal tattooing localization has several advantages. First, charcoal tattooing may remain up to 3 months in the patient [3]. Charcoal tattooing can be implemented ahead of the operation; surgeons can have some flexibility in scheduling. Second, charcoal injection for tattooing has no secondary complications, such as abscess, pain, fever; toxicity has never been reported with charcoal injection to date [13,20]. Third, no additional diagnostic instruments are needed in the operation field. The injected charcoal is easy to visualize in the surgical field and specimen. When present, it appears as a black area inside or at the periphery of the injected lesions (Fig. 2). Fourth, the practice of charcoal injection is easy and safe, because basically the same technique is used as US-guided fine-needle aspiration cytology. Several authors reported that the technical success rate was 84%-96% in the case of using charcoal for localization. In this study, there were no complications including bleeding, hematoma, abscess, and fever after charcoal injection. A dot-like marking occurred in the needle insertion site in only one case. Fifth, charcoal has no interference with the results of pathology even when having tattooed to the lesions (Fig. 3). In pathologic examination, there was no evidence of inflammatory reactions after charcoal tattooing; no polynuclear neutrophils or lymphocytes had infiltrated the charcoal tattooing areas [13]. In addition, thyroid follicular cells were not stained by charcoal tattooing.
In the present study, we reported our experience of 10 cases with US-guided charcoal tattooing localization for recurrent PTC. Our major concern was to reduce the rate of complications and to remove the recurrent lesions completely. Transient hypocalcemia developed in one patient, but no RLN injury occurred. Furthermore, all the lesions were removed completely and no remnant lesions were found in follow-up US. These results showed the technical feasibility and safety of charcoal tattooing for recurrent thyroid cancer.
In conclusion, preoperative US-guided charcoal tattooing localization for recurrent thyroid cancer seems a feasible and safe procedure for reoperation. Further evaluation is warranted in larger patients' cohorts.
Figures and Tables
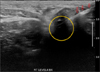 | Fig. 1Charcoal injection to localized recurrent lesions under ultrasound guidance. Needle (red arrows) tip was inserted into recurrent lymph node (yellow circle). |
 | Fig. 2Charcoal tattooing lesion was easily localized in surgical field and specimen; operation field (A) and resected mass (B). Arrows mark charcoal inside the recurrent lesion. |
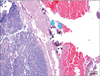 | Fig. 3The charcoal (arrows) was surrounded by collagen fibers; charcoal has no interference with results of pathology (H&E, ×100). |
References
1. Cho BY, Choi HS, Park YJ, Lim JA, Ahn HY, Lee EK, et al. Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades. Thyroid. 2013; 23:797–804.
2. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012; 44:11–24.
3. Hartl DM, Chami L, Al Ghuzlan A, Leboulleux S, Baudin E, Schlumberger M, et al. Charcoal suspension tattoo localization for differentiated thyroid cancer recurrence. Ann Surg Oncol. 2009; 16:2602–2608.
4. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Longterm outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006; 91:2892–2899.
5. Harari A, Sippel RS, Goldstein R, Aziz S, Shen W, Gosnell J, et al. Successful localization of recurrent thyroid cancer in reoperative neck surgery using ultrasound-guided methylene blue dye injection. J Am Coll Surg. 2012; 215:555–561.
6. Lefevre JH, Tresallet C, Leenhardt L, Jublanc C, Chigot JP, Menegaux F. Reoperative surgery for thyroid disease. Langenbecks Arch Surg. 2007; 392:685–691.
7. Karwowski JK, Jeffrey RB, McDougall IR, Weigel RJ. Intraoperative ultrasonography improves identification of recurrent thyroid cancer. Surgery. 2002; 132:924–928.
8. Duprez R, Lebas P, Marc OS, Mongeois E, Emy P, Michenet P. Preoperative US-guided hook-needle insertion in recurrent lymph nodes of papillary thyroid cancer: a help for the surgeon. Eur J Radiol. 2010; 73:40–42.
9. Sippel RS, Elaraj DM, Poder L, Duh QY, Kebebew E, Clark OH. Localization of recurrent thyroid cancer using intraoperative ultrasound-guided dye injection. World J Surg. 2009; 33:434–439.
10. Binyousef HM, Alzahrani AS, Al-Sobhi SS, Al SH, Chaudhari MA, Raef HM. Preoperative neck ultrasonographic mapping for persistent/recurrent papillary thyroid cancer. World J Surg. 2004; 28:1110–1114.
11. Kim WW, Kim JS, Hur SM, Kim SH, Lee SK, Choi JH, et al. Radioguided surgery using an intraoperative PET probe for tumor localization and verification of complete resection in differentiated thyroid cancer: a pilot study. Surgery. 2011; 149:416–424.
12. Kang TW, Shin JH, Han BK, Ko EY, Kang SS, Hahn SY, et al. Preoperative ultrasound-guided tattooing localization of recurrences after thyroidectomy: safety and effectiveness. Ann Surg Oncol. 2009; 16:1655–1659.
13. Mathieu MC, Bonhomme-Faivre L, Rouzier R, Seiller M, Barreau-Pouhaer L, Travagli JP. Tattooing breast cancers treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2007; 14:2233–2238.
14. Gopalakrishna Iyer N, Shaha AR. Complications of thyroid surgery: prevention and management. Minerva Chir. 2010; 65:71–82.
15. Candell L, Campbell MJ, Shen WT, Gosnell JE, Clark OH, Duh QY. Ultrasound-guided methylene blue dye injection for parathyroid localization in the reoperative neck. World J Surg. 2014; 38:88–91.
16. Triponez F, Poder L, Zarnegar R, Goldstein R, Roayaie K, Feldstein V, et al. Hook needle-guided excision of recurrent differentiated thyroid cancer in previously operated neck compartments: a safe technique for small, nonpalpable recurrent disease. J Clin Endocrinol Metab. 2006; 91:4943–4947.
17. Rubello D, Salvatori M, Casara D, Piotto A, Toniato A, Gross MD, et al. 99mTcsestamibi radio-guided surgery of locoregional 131Iodine-negative recurrent thyroid cancer. Eur J Surg Oncol. 2007; 33:902–906.
18. Rubello D, Salvatori M, Ardito G, Mariani G, Al-Nahhas A, Gross MD, et al. Iodine-131 radio-guided surgery in differentiated thyroid cancer: outcome on 31 patients and review of the literature. Biomed Pharmacother. 2007; 61:477–481.
19. Travagli JP, Cailleux AF, Ricard M, Baudin E, Caillou B, Parmentier C, et al. Combination of radioiodine (131I) and probeguided surgery for persistent or recurrent thyroid carcinoma. J Clin Endocrinol Metab. 1998; 83:2675–2680.
20. Naveau S, Bonhomme L, Preaux N, Chaput JC. A pure charcoal suspension for colonoscopic tattoo. Gastrointest Endosc. 1991; 37:624–625.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download