Abstract
Purpose
Little is known about the clinicopathological features of early mucinous gastric carcinoma (MGC). The purpose of this study was to compare the clinicopathological features and prognosis between patients with early MGC and those with early nonmucinous gastric carcinoma (NMGC).
Methods
We reviewed the records of 2,732 patients diagnosed with gastric carcinoma who were treated surgically. There were 14 patients (0.5%) with early MGC and 958 with early NMGC.
Results
Early MGC patients had a higher prevalence of elevated type (71.4%) compared with early NMGC patients (29.5%). More early MGC patients had submucosal carcinoma, compared with early NMGC patients (78.6% vs. 64.1%). The overall 5-year survival of the patients with early MGC was 97.2% as compared with 92.7% for the patients with early NMGC (P < 0.01). The statistically significant prognostic parameters influencing the 5-year survival rate according to Cox's proportional hazard regression model were: age (risk ratio, 2.22; 95% confidence interval [CI], 1.62-3.04; P < 0.01); sex (risk ratio, 1.97; 95% CI, 1.42-2.73; P < 0.01); and lymph node metastases (risk ratio, 1.88; 95% CI, 1.28-2.77; P < 0.01).
Mucinous gastric carcinoma (MGC) is a rare pathological subtype, comprising 2.4%-10% of all gastric carcinomas [1,2,3,4]. Furthermore, the incidence of early MGC is very rare and ranges from 0.2% to 1.0% at the time of diagnosis [1,3,5,6,7]. These types of tumours are detected mainly at an advanced stage and only infrequently at an early stage. Some studies have supported a worse prognosis for patients with a mucin-producing histological subtype of gastric adenocarcinoma, whereas others have reported an indolent course.
Owing to this rarity, few reports are available on the survival of patients with early MGC. Additionally, little is known about the clinicopathological features of early MGC. The purpose of this study was to describe the clinicopathological features and determine the prognosis for patients with early MGC in comparison with early non-mucinous gastric carcinoma (NMGC).
We reviewed the records of 2,732 consecutive patients diagnosed with gastric carcinoma who were treated surgically from January 1999 to December 2007 at the Department of Surgery. A total of 14 of the patients (0.5 %) had a histological diagnosis of early MGC.
We followed the criteria of the World Health Organization (WHO) classification for histological typing of gastric carcinomas in the diagnosis of MGC. All of the tissues were examined by pathologists. The clinicopathological data were analyzed according to the rules of The Japanese Research Society for Gastric Cancer [8]. Patients were evaluated based on age, sex, tumour size, tumour location, depth of invasion, macroscopic type, lymph node metastasis, tumour stage at presentation, operation type, and survival rates.
The data were analysed statistically using the chi-square test. Survival was analysed using the Kaplan-Meier method. Multivariate analysis was performed using the Cox proportional hazards model. A P-value < 0.05 indicated statistical significance.
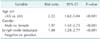
There was no significant difference in mean age between early MGC patients (52.2 years) and early NMGC patients (56.7 years) (P = 0.135). Of the 14 patients with early MGC, 11 (78.6%) were male and three (21.44%) were female. The group of 958 patients with early NMGC included 613 male (64.0%) and 345 female (36.0%) patients. There were more male than female patients in each group, but the difference in the sex ratio between early MGC and early NMGC patients was not significant (Table 1).
The mean tumour size was larger in early MGC than in early NMGC (2.39 cm vs. 2.27 cm), but this difference was not significant. Most gastric carcinomas were located in the lower third of the stomach, in both early MGC (10 cases, 71.5%) and early NMGC (634 cases, 66.2%), and the difference in tumour location was not significant (Table 1).
The prevalence of the elevated macroscopic type was higher in early MGC patients (71.4%, 10 of 14 patients) than in early NMGC patients (29.5%, 283 of 958 patients). More early MGC patients had submucosal carcinoma, compared with early NMGC patients (78.6% vs. 64.1%, P > 0.05). Depth of invasion did not differ significantly between the two groups (Table 1). There was no significant difference in nodal involvement or operative type between early MGC and early NMGC patients (P > 0.05) (Table 1).
Patients with early MGC showed a better survival rate than patients with early NMGC. The overall 5-year survival of patients with early MGC was 97.2% as compared with 92.7% for patients with early NMGC (P < 0.01) (Fig. 1). The clinicopathological variables tested in the univariate analysis are shown in Table 2. Factors influencing the 5-year survival rate were age, sex, operative type, stage at presentation, and lymph node metastasis. The statistically significant prognostic parameters influencing the 5-year survival rate according to Cox's proportional hazard regression model were age (risk ratio, 2.22; 95% confidence interval [CI], 1.62-3.04; P < 0.01); sex (risk ratio, 1.97; 95% CI, 1.42-2.73; P < 0.01); and lymph node metastases (risk ratio, 1.88; 95% CI, 1.28-2.77; P < 0.01) (Table 3).
The histological classification of gastric carcinoma is controversial [9]. The most common systems in use include the WHO, the Lauren, and Ming classifications. But, no histological system is ideal because tumors are not uniform and show considerable overlap of histological patterns.
Detailed knowledge of the clinicopathological features and prognostic factors for early MGC is important for diagnosis and treatment, but little is known about the clinicopathological features of early MGC patients. Some reports have described the rarity of early gastric carcinoma with mucinous histology. MGC is a rare histological type, representing 2.4%-10% of all gastric carcinomas [1,2,3,4], and early MGC is even rarer with an incidence of 0.2%-1.0% of all gastric carcinomas [1,3,5,6,7]. However, in one study, mucinous histology was identified in 10.7% of 168 cases of early gastric carcinoma [10]. It has also been reported that the incidence of early cases was only 20.0% in MGC, while among other pathological types of carcinoma, 44.6% were diagnosed at an early stage [4]. In contrast to these reports, some investigators have reported a similar incidence of early MGC and early NMGC cases (10.4% vs. 10.8%) [11]. In the present study, early MGC cases were 14.4% of all early gastric carcinoma cases.
Early MGC is characterized by its macroscopic appearance. In the present study, early MGC patients had a higher prevalence of the elevated macroscopic type (71.4%, 10 of 14 patients) compared with early NMGC patients (29.5%, 283 of 958 patients). Others have also reported that early MGC tumours are macroscopically more elevated than early NMGC tumours [7,12].
Microscopically, MGC that is restricted to the mucosa is very rare. One study reported that among 337 early gastric carcinomas, seven were MGC, and only one of these was limited to the mucosa; the other six cases showed invasion of the submucosa [13]. Other researchers have found similar results [5,6,7,12]. It is presumed that MGC is initially an ordinary adenocarcinoma, and as it progresses and invades the submucosa or deeper layers in the gastric wall, the intraluminal extension of mucin decreases and intraluminal accumulation of the mucin pool increases to an extent that is sufficient for diagnosis. This may explain the rarity of MGC that is restricted to the mucosa [7,12,14]. In our study, only three of 14 cases of early MGC were confined to the mucosa.
Several studies have shown that the outcome of patients with MGC is worse than that of patients with NMGC [1,7]. Patients with MGC may have poor outcomes because of its detection at an advanced stage and not because of its mucinous histology [6,7,12]. Several hypotheses have been proposed to explain why MGC is almost always diagnosed at a late stage [15]. In contrast, some investigators have found no significant difference between the overall survival rates of early MGC and early NMGC [11,13]. Furthermore, some studies have reported that the surgical outcome is better for patients with early MGC than for patients with early NMGC [7,12]. In the present study, we also found a better survival rate for early MGC than for early NMGC. These findings support the idea that the poor prognosis for MGC patients is not associated with the mucinous histology, but rather with the advanced tumour stage.
Histological type as a prognostic factor is still controversial [16]. It has been stated that mucinous histological type itself is not an independent prognostic factor in patients with MGC [12,14], and our results confirm this statement for patients with early MGC. In the present study, multivariate analysis showed that three factors were independent, statistically significant parameters associated with survival: age, sex, and lymph node metastasis.
In conclusion, patients with early MGC had a better prognosis than those with early NMGC in the present study. Mucinous histology itself appears not to be an independent prognostic factor. Thus, early detection is important for improving the prognosis for patients with gastric carcinoma regardless of tumour histology.
Figures and Tables
Fig. 1
Survival curves of early mucinous gastric carcinoma (MGC) and early nonmucinous gastric carcinoma (NMGC; 5-year survival rate: 97.2% vs. 92.7%, P = 0.001).

Table 1
Clinicopathologic features early mucinous gastric carcinoma (MGC) and early nonmucinous gastric carcinoma (NMGC)

References
1. Adachi Y, Mori M, Kido A, Shimono R, Maehara Y, Sugimachi K. A clinicopathologic study of mucinous gastric carcinoma. Cancer. 1992; 69:866–871.
2. Wu CY, Yeh HZ, Shih RT, Chen GH. A clinicopathologic study of mucinous gastric carcinoma including multivariate analysis. Cancer. 1998; 83:1312–1318.
3. Kawamura H, Kondo Y, Osawa S, Nisida Y, Okada K, Isizu H, et al. A clinicopathologic study of mucinous adenocarcinoma of the stomach. Gastric Cancer. 2001; 4:83–86.
4. Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, et al. Clinicopathologic characteristics and surgical outcomes of mucinous gastric carcinoma. Ann Surg Oncol. 2006; 13:836–842.
5. Hyung WJ, Noh SH, Shin DW, Yoo CH, Kim CB, Min JS, et al. Clinicopathologic characteristics of mucinous gastric adenocarcinoma. Yonsei Med J. 1999; 40:99–106.
6. Lim SW, Kim DY, Kim YJ, Kim SK. Clinicopathologic features of mucinous gastric carcinoma. Dig Surg. 2002; 19:286–290.
7. Yasuda K, Shiraishi N, Inomata M, Shiroshita H, Ishikawa K, Kitano S. Clinicopathologic characteristics of early-stage mucinous gastric carcinoma. J Clin Gastroenterol. 2004; 38:507–511.
8. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English edition. Gastric Cancer. 1998; 1:10–24.
9. Davis GR. Neoplasms of the stomach. In : Sleisenger MH, Fordtrans JS, editors. Gastrointestinal disease. Philadelphia: Saunders;1993. p. 763–768.
10. Caruso RA. The histogenesis of mucinous adenocarcinoma of the stomach from observations in early gastric cancer. Ann Diagn Pathol. 1999; 3:160–164.
11. Zhang M, Zhu GY, Zhang HF, Gao HY, Han XF, Xue YW. Clinicopathologic characteristics and prognosis of mucinous gastric carcinoma. J Surg Oncol. 2010; 102:64–67.
12. Adachi Y, Yasuda K, Inomata M, Shiraishi N, Kitano S, Sugimachi K. Clinicopathologic study of early-stage mucinous gastric carcinoma. Cancer. 2001; 91:698–703.
13. Yin C, Li D, Sun Z, Zhang T, Xu Y, Wang Z, et al. Clinicopathologic features and prognosis analysis of mucinous gastric carcinoma. Med Oncol. 2012; 29:864–870.
14. Choi MG, Sung CO, Noh JH, Kim KM, Sohn TS, Kim S, et al. Mucinous gastric cancer presents with more advanced tumor stage and weaker β-catenin expression than nonmucinous cancer. Ann Surg Oncol. 2010; 17:3053–3058.
15. Ma J, De Boer WG, Nayman J. Intestinal mucinous substances in gastric intestinal metaplasia and carcinoma studied by immunofluorescence. Cancer. 1982; 49:1664–1667.
16. Kim JP. Results of surgery 6,589 gastric cancer patients indicating immunochemosurgery as being the best multimodality treatment for advanced gastric cancer. In : Nishi M, Ichikawa H, Nakajima T, Maruyama K, Tahara E, editors. Gastric cancer. Tokyo: Springer;1993. p. 358–377.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download