Abstract
Sparganosis of the breast is an uncommon disease, but should be considered in the differential diagnosis of unusual and suspicious breast masses. A history of ingesting contaminated water and direct ingestion of snakes and frogs may help in differential diagnosis of the mass. Complete surgical removal is the treatment of choice and provides a definite diagnosis. We report a case of multiple axillary lymph node sparganosis. It was first considered as an axillary metastasis of breast cancer because a newly axillary mass appeared in follow-up radiologic study after neoadjuvant chemotherapy. We performed curative breast cancer surgery and sparganosis was confirmed by extracting the worm during axillary dissection.
Sparganosis is a rare infection disease caused by sparganum, a plerocercoid tapeworm larva of genus Spirometra. In humans, it is accidentally acquired by ingestion of larvae-containing water or by eating raw snakes and frogs. The larvae are commonly found in the abdomen, urogenital organs, extremities, central nervous system, chest, and orbital region [1]. The breast is a rare site of infection. The most common clinical manifestation of sparganosis is a migrating subcutaneous mass. Clinically and radiologically, the mass may mimic a neoplasm. It is very difficult to diagnose preoperatively in most cases [2]. We report an interesting case of axillary sparganosis involving multiple axillary lymph nodes in a breast cancer patient receiving neoadjuvant chemotherapy that was confused as axillary metastasis.
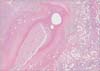
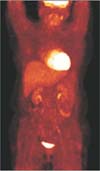
A 52-year-old woman visited Konkuk University Medical Center to have an examination on her right-side breast mass, palpable from one month prior. She said that the right-side breast mass had been noticed one month before, which then grew bigger. Pain did not accompany, and nipple discharge did not exist. An image-guided core needle biopsy showed invasive ductal carcinoma. Initial breast MRI revealed a 5-cmsized regional enhancement at the right upper breast with negative findings in the right axilla (Fig. 1A). And 18F-fluoro-2-D-glucose (18FDG) PET/CT demonstrated a hypermetabolic mass with SUVmax 13.0 at the upper outer quadrant of the right breast without significant hypermetabolism at the right axilla (Fig. 1B). The patient underwent neoadjuvant chemotherapy consisting of doxorubicin and cyclophosphamide, every 3 weeks. After three cycles, efficacy was assessed. The breast MRI showed a decreased size and volume of cancer from 5 cm to 3 cm and newly appearing enlarged lymph nodes that were considered metastasis at the right axilla (Fig. 2A). Also, 18FDG PET/CT showed a decreased size and activity of cancer and newly developed hypermetabolic lymph nodes with SUVmax 7.2 at the right axilla (Fig. 2B). So, we concluded the breast cancer progression and decided upon curative surgical treatment with breast-conserving surgery by right axillary dissection. In the surgical field, whitish and shiny worms were found inside the lymph nodes, which confirmed as spargana (Fig. 3). The histologic findings confirmed degenerative parasitic organisms with granulomatous chronic inflammation and eosinophilic infiltration (Fig. 4). And there was no metastasis in the 15 lymph nodes.
The patient completed 3-cycle postoperative chemotherapy, and radiation therapy. Herceptin was administered every 3 weeks, due to ER(-). PR(-), c-erb-B2(3+). It has been 15 months since the first treatment, and the patient is in good shape without recurrence (Fig. 5).
Sparganosis caused by the migrating procercoid larvae of Diphyllobothrium mansoni is an extremely rare disease. The most common route of infection is via ingestion of contaminated water containing procercoids, which can penetrate into the intestine and migrate to the muscle or subcutaneous tissue. The second route is from ingestion of raw or partially cooked fish, frogs, snakes, and chickens. Infection in this case also occurs from migration of the procercoid through the intestine. The third route of infection may occur from poultice applications that may be infested with Cyclops containing procercoids that are utilized to open wounds or eyes [3]. In the past, the typical route of infection was direct oral consumption of plero-cercoid-infected reptiles or amphibians, most frequently an intermediary host such as snakes or frogs or eating uncooked mammals such as pigs [4]. It is possible that economic development and advancement in sanitation have influenced routes, sites of infection, and latent period in recent times. Our patient denied eating either snakes or frogs. But, only once, she admitted to drinking water from a spring when climbing a mountain. Therefore, a history of drinking impure water should be considered a cause of axillary sparganosis.
Spargana larvae can be found in any part of the human body and have a preference for subcutaneous involvement and migration. In the extramammary organs, sparganosis most frequently manifests as a migrating subcutaneous nodule and it presents clinically with vague or indeterminate symptoms [5]. The clinical features of breast sparganosis were reported an as indolent palpable mass that was similar to malignancy with the absence of any inflammatory reaction such as a warm sensation or painful swelling [6]. Currently, the majority of suspicious or indeterminate breast lesions undergo core needle biopsy. However, preoperative biopsy procedures may result in fragmenting the worms, thus preventing complete surgical removal and possibly promoting recurrences. The definitive diagnosis of sparganosis can only be performed by excisional biopsy and by confirmation of the existence of a sparganum larva. This condition is too difficult to diagnose [7]. In our case, sparganosis was revealed in a newly developed axillary mass with follow-up MRI and 18FDG PET/CT after neoadjuvant chemotherapy. and it was mistaken for breast cancer progression, axillary metastasis. It was an unusual case, because the main breast cancer had decreased having partial response to chemotherapy. We decided on curative surgical treatment; breast-conserving surgery with axillary dissection. Eventually, the diagnosis was established at surgery with identification of the worm in the axillary lymph nodes. There was no lymph node metastasis.
When sparganum is ingested, it penetrates the intestinal wall to reach the peritoneal cavity and begins to migrate systemically. The longevity of sparagana is thought to be under 1 year. However, some articles have reported finding a live worm after more than 10 years [4]. We experienced an interesting case of sparganosis that had been latent for only one month without symptoms. In this case, we concluded that the sparganum migrated to the axillary lymph nodes and the immunosuppressant therapy might have caused lymphadenopathy. Although the exact length of latency is not specific in most published articles, our case could be the shortest known latent period of spargnosis. The short-term survival of the parasites during chemotherapy may result from their immunomodulatory ability with respect to the host. Axillary and inguinal sparganosis in an immunosuppressed patient with lymphoma who had received allo-peripheral stem cell transplant and cyclosporine therapy has been reported [8]. Further research is required in order to understand the role of sparganum as a valuable model for alleviating symptoms of immune-mediated disease.
Human sparganosis is a rare disease. And the infection route, latent period, and symptoms of the disease are changing with economic development. In this respect, surgeons should be carefully taking patient history in order to make a differential diagnosis. In this report, we speculate that the extremely short period of spargana could have been under one month with a parasitic infection occurring in the right axillary lymph nodes following neoadjuvant chemotherapy.
Figures and Tables
 | Fig. 1Initial breast imaging. (A) Breast MRI shows 5-cm-sized regional enhancement at right upper breast with negative finding in right axilla. (B) 18F-fluoro-2-D-glucose positron PET-CT shows hypermetabolic lesions in right upper breast without significant hypermetabolism at right axilla. |
 | Fig. 2Follow-up breast imaging after 3 cycles of neoadjuvant chemotherapy. (A) Breast MRI shows interval decrease of nonmass-like enhancement in right breast and newly appeared enlarged lymph nodes at right axilla. (B) 18F-fluoro-2-D-glucose PET-CT shows a decreased size and activity of right breast cancer and newly developed hypermetabolic lymph nodes at right axilla. |
 | Fig. 3Gross appearance of whitish flat sparganum extracted from the axillary lymph nodes by surgeon in the operating room. |
References
1. Park JH, Chai JW, Cho N, Paek NS, Guk SM, Shin EH, et al. A surgically confirmed case of breast sparganosis showing characteristic mammography and ultrasonography findings. Korean J Parasitol. 2006; 44:151–156.
2. Kim HY, Kang CH, Kim JH, Lee SH, Park SY, Cho SW. Intramuscular and subcutaneous sparganosis: Sonographic findings. J Clin Ultrasound. 2008; 36:570–572.
3. Cho JH, Lee KB, Yong TS, Kim BS, Park HB, Ryu KN, et al. Subcutaneous and musculoskeletal sparganosis: imaging characteristics and pathologic correlation. Skeletal Radiol. 2000; 29:402–408.
4. Koo M, Kim JH, Kim JS, Lee JE, Nam SJ, Yang JH. Cases and literature review of breast sparganosis. World J Surg. 2011; 35:573–579.
5. Chan AB, Wan SK, Leung SL, Law BK, Lai DP, Ip M, et al. Sparganosis of the breast. Histopathology. 2004; 44:510–511.
6. Chang YK, Kim KC, Cho HJ. Sparganosis in the female breast. J Korean Surg Soc. 2000; 58:285–287.
7. Sarukawa S, Kawanabe T, Yagasaki A, Shimizu A, Shimada S. Case of subcutaneous sparganosis: use of imaging in definitive preoperative diagnosis. J Dermatol. 2007; 34:654–657.
8. Yoon HS, Jeon BJ, Park BY. Multiple sparganosis in an immunosuppressed patient. Arch Plast Surg. 2013; 40:479–481.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download