Abstract
Intra-abdominal fibromatosis (IAF) may arise either sporadically or in association with familial adenomatous polyposis. The characteristics of fibromatosis are slow-growth, benign histological features, and aggressive local invasion. Surgery remains a reasonable first treatment option. Here, we report 2 cases of a phenomenon rarely described in published literature, IAF after gastrectomy for gastric cancer. Intra-abdominal masses were found during the routine follow-up period in a 50-year-old man who had received a radical subtotal gastrectomy for early gastric cancer. Two mesenteric masses were detected in the upper abdomen by CT and were excised completely along with segments of the jejunum. Another intra-abdominal mass was found in 60-year-old man who had received a radical total gastrectomy for advanced gastric cancer. A 4.2-cm-sized mass was detected in the periumbilical region by follow-up CT and was excised completely along with a segment of the ileum.
Fibromatosis (desmoid tumors) are rare soft tissue tumors of mesenchymal origin that arise from musculoaponeurotic structures. Generally, the locations of fibromatosis are categorized as extra-abdominal (extremities), abdominal wall, and intra-abdominal (the bowel wall and mesentery). Intra-abdominal fibromatosis (IAF) may arise sporadically or in association with familial adenomatous polyposis (FAP). Fibromatosis tumors exhibit slow-growth, benign histological features, and aggressive local invasion without metastasis [1]. Surgery remains a reasonable first treatment for operable tumors but may lead to high rates of morbidity and recurrence. Therefore, some cases of FAP-associated fibromatosis may be treated with drugs as a first option. However, gastric cancer-associated fibromatosis are very rare, difficult to diagnosis, and difficult to treat given the choice between surgical or pharmacological interventions. Komatsu et al. [2] first reported IAF after gastrectomy for gastric cancer. Herein, we report 2 cases of complete excision of IAF after gastrectomy for gastric cancer.
A 50-year-old man was admitted after nonpalpable intra-abdominal masses were detected by CT. This patient had received radical subtotal gastrectomy with gastrojejunostomy and jejunojejunostomy 2 years prior to admission. The histologic type was poorly differentiated adenocarcinoma and the stage was pT1N1M0 according to the American Joint Committee on Cancer, 7th edition. At the time of gastrectomy, no other intra-abdominal masses were found. After curative resection without adjuvant chemotherapy, he was checked routinely via biannual CT and annual endoscopy. Two years after gastrectomy, 2 jejunal mesenteric masses were detected by CT. The sizes were approximately 5 and 6 cm in diameter. Exploratory laparotomy was performed to confirm the pathological characteristics of the masses and to determine whether the masses were reflective of recurrent gastric cancer or of benign tumors (e.g., gastrointestinal stromal tumors [GISTs]). One mass detected in the jejunal mesentery near the gastrojejunostomy site was excised completely with the jejunal segment (Fig. 1). Additional gastrojejunostomy was performed because the previous gastrojejunostomy site was involved in the resected segment. Another tumor was detected in the jejunal mesentery 100 cm below the gastrojejunostomy site and was excised completely with the jejunal segment (Figs. 2, 3). Pathologically, the 2 tumors were diagnosed as mesenteric fibromatosis. No recurrence or metastasis had occurred during the 5-year follow-up period.
A 60-year-old man was admitted with a nonpalpable abdominal mass. This patient had undergone radical total gastrectomy for advanced gastric cancer 23 months prior to admission. The histologic type was mucinous adenocarcinoma and the stage was pT2N2M0. Additionally, peritoneal seeding or intra-abdominal masses were not detected during an initial laparotomy. Adjuvant chemotherapy was performed. He was checked routinely via biannual CT and an upper gastrointestinal series. Twenty-three months after surgery, a 4.2-cm-sized mass was detected in the periumbilical region by CT (Fig. 4). The radiologist recommended 18F-fluorodeoxyglucose PET/CT, which revealed a mild hypermetabolic mass in the periumbilical region that appeared to be metastatic (Fig. 5). Exploratory laparotomy was performed and the tumor was detected 30 cm above the ileocecal valve in the ileal mesentery. This mass was excised completely with the ileal segment. Macroscopically, the tumor was poorly demarcated and firm, measuring 6.0 cm × 5.8 cm × 2.3 cm (Fig. 6). Microscopically, the tumor showed proliferation of spindle-shaped cells with uniform cytologic features and focal dense collagen deposition (Fig. 7). Immunohistochemically, the tumor cells showed diffuse b-catenin nuclear staining (Fig. 8), but negative reactivity for CD34 and CD117. The tumor was diagnosed as a mesenteric fibromatosis. The patient recovered without complication and no recurrence or metastasis had occurred during the 3 month follow-up period.
Fibromatosis refers to a group of rare soft tissue tumors of fibroblastic origin that can occur in sites including the extremities, abdominal wall, and intestines. Based on location, fibromatosis can be subdivided into extra-abdominal, abdominal wall, and IAF. The pathogenesis of IAF is unclear but may involve germline mutations of the APC gene, sex hormones, and traumatic injuries, including surgery. Although IAF can occur sporadically in rare cases, about 68%-86% of IAF and abdominal wall fibromatosis in FAP occur after abdominal surgery [3]. The incidence of fibromatosis is higher in females and may be attributable to the abnormal metabolism of estrogen, which has been implicated in the pathogenesis of sporadic fibromatosis [4].
CT is most commonly used to investigate IAF, which may appear as a hypervascular soft tissue mass that resembles a GIST. However, IAF can be differentiated from GIST on a CT image by ovoid or irregular contours, homogeneous enhancement, and an absence of central necrosis [5]. Magnetic resonance imaging is considered to be a more advantageous method in assessing extra-abdominal fibromatosis.
The appropriate treatment of IAF remains a subject of controversy. In asymptomatic patients, simple observation may be a suitable choice. Unfortunately, complete resection may be difficult in many cases due to deep infiltration and the absence of a capsule. However, treatment is required for symptomatic patients and/or those with tumors that display a rapid growth pattern. Surgery remains the first treatment option for patients with locally circumscribed fibromatosis [6]. Radiotherapy after surgery or radiotherapy alone has been used for improvement of local control. In fact, a meta-analysis of 22 studies reported that radiotherapy after surgery demonstrated significantly better control after both R0 and R1 resections [7].
Nonsurgical modalities for the treatment of unresectable IAF include nonsteroidal anti-inflammatory drugs (NSAIDs), antiestrogens (tamoxifen or toremifene), and cytotoxic chemotherapy. Sulindac is the most commonly used drug for the treatment of FAP-associated fibromatosis and the results of 1 study demonstrated an overall response rate of 57% in 14 FAP patients [4]. The efficacy of NSAIDs has been demonstrated in small cases, however, most cases showed variable responses [4]. The incidence of IAF increases during and after pregnancy and this natural history of the disease forms the basis for antihormonal therapy. Janinis et al. [8] reported that antihormonal therapy in fibromatosis resulted in stable disease or regression. The use of cytotoxic agents for the treatment for IAF is supported by the efficacy of doxorubicin combined with other agents (dacarbazine or cyclophosphamide and vincristine). The overall response rate is approximately 40%-50%, although severe side effects such as cardiotoxicity or myelotoxicity have been reported [4]. As a result, cytotoxic chemotherapy is reserved for those that are nonoperable or nonresponsive to other treatments. IAF is often mistaken for GIST or other spindle cell neoplasms, as they are all macroscopically solid localized tumors that originate in the mesentery. However, they can be distinguished pathologically, as GISTs are found to be immunopositive for CD114, CD34, and DOG-1, while IAF are found to be immunopositive for b-catenin mutations restricted to exon 3 of CTNNB1 [9].
In conclusion, we experienced 2 cases of mesenteric fibromatosis resembling GIST or metastatic tumors. All tumors were excised completely and no recurrence occurred. Although some cases of FAP-associated fibromatosis may be treated initially with drugs, gastric cancer-associated fibromatosis are very rare, difficult to diagnosis, and without consensus regarding a preferred treatment approach (surgery or pharmacotherapy). Therefore, exploratory laparotomy followed by complete excision may be an appropriate strategy for those patients with a mesenteric mass detected after gastrectomy for gastric cancer.
Figures and Tables
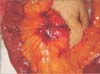
Fig. 1
First tumor in the mesentery of the jejunum near the gastrojejunostomy site (black arrow, mesenteric mass; blue arrow, gastrojejunostomy site).
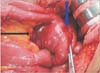
Fig. 2
Second tumor in the mesentery of the jejunum (black arrow, mesenteric mass; red arrow, jejunojejunostomy site).
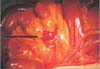
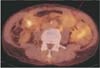
Fig. 4
A 4.2-cm-sized mass in the periumbilical region by CT: (A) horizontal view (red arrow, mesentery mass), (B) coronal view (red arrows, mesentery mass).
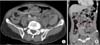
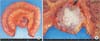
Fig. 6
Mesenteric mass and ileum excised completely. (A) resected specimen. (B) cross-sectional view.
References
1. Lewis JJ, Boland PJ, Leung DH, Woodruff JM, Brennan MF. The enigma of desmoid tumors. Ann Surg. 1999; 229:866–872.
2. Komatsu S, Ichikawa D, Kurioka H, Koide K, Ueshima Y, Shioaki Y, et al. Intra-abdominal desmoid tumor mimicking lymph node recurrence after gastrectomy for gastric cancer. J Gastroenterol Hepatol. 2006; 21:1224–1226.
3. Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg. 1996; 83:1494–1504.
4. Sturt NJ, Clark SK. Current ideas in desmoid tumours. Fam Cancer. 2006; 5:275–285.
5. Zhu H, Chen H, Zhang S, Peng W. Intra-abdominal fibromatosis: differentiation from gastrointestinal stromal tumour based on biphasic contrast-enhanced CT findings. Clin Radiol. 2013; 68:1133–1139.
6. Kasper B, Strobel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011; 16:682–693.
7. Tolan S, Shanks JH, Loh MY, Taylor B, Wylie JP. Fibromatosis: benign by name but not necessarily by nature. Clin Oncol (R Coll Radiol). 2007; 19:319–326.
8. Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol. 2003; 14:181–190.
9. Huss S, Nehles J, Binot E, Wardelmann E, Mittler J, Kleine MA, et al. β-catenin (CTNNB1) mutations and clinicopathological features of mesenteric desmoid-type fibromatosis. Histopathology. 2013; 62:294–304.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download