Abstract
Purpose
The aim of this study is to know whether silibinin has an anticancer effect on triple negative breast cancer xenograft model using MDA-MB-468 cells.
Methods
To establish the xenograft model, we injected the MDA-MB-468 cells into female Balb/c-nude mice. After establishing a xenograft model, oral silibinin was administered to the tested mice in the way of 200 mg/kg for 45 days. The difference of mean tumor volume between silibinin fed mice and control mice was analyzed. The epidermal growth factor receptor (EGFR) phosphorylation in MDA-MB-468 cells was analyzed by Western blotting. The expression of VEGF, COX-2, and MMP-9 genes in tumor tissue was analyzed by real-time polymerase chain reaction (PCR).
Results
In the xenograft model using MDA-MB-468 cells, we found that oral administration of silibinin significantly suppressed the tumor volume (silibinin treated mice vs. control mice; 230.3 ± 61.6 mm3 vs. 435.7 ± 93.5 mm3, P < 0.001). The phosphorylation of EGFR in MDA-MB-468 cells was inhibited by treatment with 50 µg/mL of silibinin. In real time-PCR analysis of tumor tissue obtained from sacrificed mice, the gene expression of MMP-9, VEGF, and COX-2 was 51.8%-80% smaller in silibinin group than that of control group and we can also verify the similar result using Western blotting analysis.
Conclusion
We verified that silibinin had anticancer effect on xenograft model of MDA-MB-468 cells in the way of preventing the phosphorylation of EGFR and eventually suppressed the production of COX-2, VEGF, and MMP-9 expression. Finally, the tumor volume of xenograft models was decreased after administration of Silibinin.
While the traditional criteria for classification of breast cancer were based on type of tumor, nuclear grade, tumor size, and presence or absence of lymph node metastasis, breast cancer has recently been classified into several subtypes based on the molecular profiling according to the expression of mRNA. Of the subtypes of breast cancer by gene expression profiling, the basal-like subtype is classified as the closest type to triple negative breast cancer, because expression of the erbB-2 receptor, the estrogen receptor, and the progesterone receptor did not appear and because 80% of basal-like subtypes are expressed as triple negative breast cancer [1]. Triple negative breast cancer accounts for 15%-20% of all breast cancer subtypes and these subtypes are important because the prognosis for them is poor compared to other subtypes of breast cancer due to the characteristics of triple negative breast cancer, that is, the absence of targets for targeted therapy and hormone receptors and aggressiveness [1,2]. Currently, the treatment of triple negative breast cancer is mainly dependent upon chemotherapy, however, despite the use of chemotherapy, it has been reported that the prognosis is still not good compared to other types of breast cancers. Therefore, many studies have been conducted to discover a target therapy for triple negative breast cancer and to develop new therapeutic drugs, but the performance of those therapeutic options is still unsatisfactory. Silibinin is a polyphenolic flavonoid extracted from silybum marianum seeds and it is known as antioxidant that has a variety of pharmacological effects [3]. In 2006, a study conducted by the University of Colorado's Singh et al. [4], found that if mice with artificially induced lung cancer were administered silibinin, the occurrence and growth of the lung cancer was inhibited and peritumoral angiogenesis was also inhibited. In another study, Jiang et al. [5] found that, when MDA-MB-468 breast cancer cells are treated with 50 µg/mL to 100 µg/mL of silibinin, expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2) is reduced by about 90% compared to when the cells are not treated with silibinin, thereby showing the antiangiogenic effect of silibinin for breast cancer cells. It has also been recently reported that silibinin has the anticancer effect of preventing proliferation of cancer by inhibiting progression of the cell cycle and cell proliferation of prostate cancer and hepatoma and promoting apoptosis of cancer cells [6,7].
The findings from this current study confirmed the mechanism that silibinin causes arrest of the G2/M phase and inhibits the growth of breast cancer cells through down regulation of cyclin B1 and cdc2 proteins and the up-regulation of p21 protein by using the MDA-MB-231 cell line, one of the triple negative breast cancer cell lines. Through a previous in vitro experiment, we have also confirmed that silibinin inhibits expression of VEGF and MMP-9 affecting invasiveness and metastasis of cancer cells by using MDA-MB-231 triple negative breast cancer cells [8,9]. As shown above, based on our previous in vitro experimental results that silibinin has an anticancer effect for triple negative breast cancer cells, this study aimed to determine if silibinin has anticancer effects on a triple negative breast cancer xenograft model using MDA-MB-468, the triple negative breast cancer cells.
The MDA-MB-468 (American Type Culture Collection, Manassas, VA, USA) human breast cancer cell line was cultured in an environment of 95% air and 5% CO2 at 37℃ and in 10% fetal bovine serum (FBS), 2mM glutamine, 100-IU/mL penicillin and 100-µg/mL streptomycin added to Rosewell Park Memorial Institute-1640 medium (Life Technology, Rockville, MD, USA), and each cell was maintained in a culture medium that did not include FBS for 24 hours before the experiment.
Silibinin was used to treat the MDA-MB-468 cells as follows: First, the MDA-MB-468 cells were preserved in cell culture medium without FBS for 24 hours and then the cells were maintained again in a culture medium that did not contain FBS that was treated with 25 µg/mL and a 50 µg/mL concentration of silibinin for 24 hours. In addition, before treating with epidermal growth factor (EGF) (R&D System, Minneapolis, MN, USA), silibinin was pretreated for 1 hour and then EGF and silibinin were treated at the same time and preserved for 24 hours.
Western blotting analysis was conducted in order to analyze the degree of protein expression by using a MDA-MB-468 cytolysate. First, we boiled the protein in a Laemmli sample buffer for about 5 minutes and then it was electrophoresed in 8% sodium dodecyl sulfate polyacrylamide gel to pass the protein to the polyvinylidene fluoride membrane, and then the movement of the nonspecific protein was blocked by using 10% skim milk for 15 minutes in Tris buffered saline (TBS) that also included 0.01% Tween-20. All the blots were cultured for about 12 hours at 4℃ with anti-t-epidermal growth factor receptor (EGFR), p-EGFR (R&D System, Minneapolis, MN, USA) primary antibody and then washed, three times, in TBS/T and reacted again in TBS/T buffer solution with antirabbit peroxidase-conjugated antibody (1/2,000 dilution), a secondary antibody. After being cultured for about 1 hour at room temperature, the blots were washed in TBS/T buffer solution three times and ECLplus (Amersham, Buckinghamshire, UK) reagent was used for development.
In order to establish a nude mice xenograft model, we used Balb/c-nude mice (Charles River Japan, Yokohama, Japan) aged 5-6 weeks, weighing about 18-22 g. All the mice were raised in a pathogen-free environment within the animal laboratory under a 12-hour light/12-hour dark cycle. A standard rodent chow diet (Laboratory Rodent Diet 5001, LabDiet, St. Louis, MO, USA) was supplied by using libitum.
MDA-MB-468 5 × 106 cells/0.2 mL were injected into subcutaneous tissue on the thigh of each mouse and the tumor size of each mouse was measured 10 weeks after cell transplantation; then, the mice were randomly divided into two groups, control mice and silibinin-treated mice, and each group consisted of five mice. Next, 0.5% carboxymethyl cellulose was administered to the control mice every day and 200 mg/kg/day of silibinin was administered to the silibinin-treated mice every day (Fig. 1). The tumor size of the control mice and the silibinin-treated mice was measured using digital calipers at the time of administrating the drug and after three days, every week, and the volume was measured by measuring the minor axis and the major axis of the tumor. We measured the weights of the mice at the same time that the tumor volume was measured and, finally, the tumor size was measured 45 days after the drug administration. Euthanasia was done for the control mice and the silibinin-treated mice by using the cervical dislocation method and the size and weight of the tumor excised from each of the mice was once again measured. The experiment was repeated twice.
We separated the tumor tissue subcutaneously transplanted in the Balb/c-nude mice and placed the separated tumor tissue and Trizol reagent (Invitrogen, Carlsbad, CA, USA) in a microtube for homogenization and then we extracted RNA in the method presented by the manufacturer. The extracted RNA was quantified and then 1 µg of RNA from each tumor tissue sample was synthesized with cDNA by using the cDNA Synthesis Kit (Fermentas, Hanover, MD, USA). We compared and analyzed the expression of genes by using synthesized cDNA. The primer sequence of the genes is as follows: β-actin (5': TCACCATTGGCAATGAGCGGTT, 3': AGTTTCGTGGATGCCACAGGACT), MMP-9 (5': TTGACAGCGACAAGAAGTGG, 3': GCCATTCACGTCGTCCTTAT), COX-2 (5': GCAGTTGTTCCAGACAAGCA, 3': GGTCAATGGAAGCCTGTGAT), and VEGF (5': GGGCCTCCGAAACCATGAAC, 3': CAAGGCTCCAATGCACCCAA). Finally, polymerase chain reaction (PCR) was carried out by using the 7900HT Fast Real-Time PCR system (Applied Biosystems, Life Technologies, Grand Island, NY, USA). Reference genes were normalized by using β-actin and each mRNA expression was identified. The relative quantification = 2∧(p-ΔΔCT), here ΔΔCT = (CT,Target-CT,actin)Treatment-CT,Target-CT,actin)Control.
Student t-test was used for statistical information about the difference in each mean tumor size that occurred in the control mice and the silibinin-treated mice, and each result was shown as mean ± standard error. All P-values were subjected to a two-tailed test and less than 0.05 of P-value was deemed to be statistically significant. The analysis was performed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA).
Based on the previous report that expression of EGFR accounts for about 60% of all triple negative breast cancer, the expression of EGFR families in MDA-MB-231 and MDA-MB-468 cells was analyzed. As shown in Fig. 2, the Western blotting experiment showed that, in the case of the MDA-MB-231 cells, only EGFR is expressed, and in the case of the MDA-MB-468 cell line, HER3 and EGFR are expressed.
In order to verify the anticancer effect of silibinin in the animal model, MDA-MB-468 cells were injected into the subcutaneous tissue of mice and implanted for 10 weeks and then 200 mg/kg/day of silibinin was orally administered to silibinin-treated mice every day. It was found that the mean size of the tumor in the silibinin-treated mice began to show a difference in tumor volume on the 10th day of silibinin administration compared to the mean size of the tumor in the control mice. The mean volume of the tumor in the silibinin-treated mice was 230.3 ± 61.6 mm3 the 45th day after silibinin administration and the tumor volume was reduced by about 52.8% (P < 0.001) compared to the mean tumor volume of the control mice; the average volume of the tumor in the control mice was 435.7 ± 93.5 mm3 (Fig. 3). In addition, throughout the entire period of the animal experiment, there was no difference in mean weight between the silibinin-treated mice and the control mice and there was no drug toxicity by silibinin.
In order to determine whether silibinin inhibited the EGF-EGFR signaling pathway, silibinin was first pretreated in the MDA-MB-468 cells for about 60 minutes at concentrations of 25 µg/mL and 50 µg/mL, and then it was stored for 24 hours with EGF. As shown in Fig. 4, it was found that while phosphorylation of EGFR increases after EGF treatment, expression of phosphorylated EGFR decreases in proportion to the silibinin concentration if treated with silibinin (Fig. 4).
By using tumor tissue taken from the silibinin-treated mice and the control mice, semiquantitative real-time PCR was carried out to see if silibinin had a regulatory effect at the mRNA level of MMP-9, VEGF, and COX-2 (Fig. 5). Real-time PCR results showed that the mRNA levels of MMP-9, VEGF, and COX-2 in the silibinin-treated mice decreased with statistical significance compared to the MMP-9, VEGF, and COX-2 of the tumor tissue taken from the control mice (P < 0.001). Similar to the real-time PCR results, COX-2 and VEGF protein expression was also reduced in the silibinin-treated mice in Western blotting (Fig. 5). As a result, silibinin turned out to inhibit COX-2, VEGF, and MMP-9 protein expression by adjusting the mRNA levels of MMP-9, VEGF, and COX-2. As shown above, silibinin is estimated to inhibit the growth and metastasis of breast cancer through inhibition of COX-2, MMP-9, and VEGF protein expression.
The prognosis of triple negative breast cancer, which currently accounts for about 15%-20% of all breast cancer, is known to be less favorable compared to other subtypes of breast cancer [1,2]. The important reasons for the poor prognosis of triple negative breast cancer are known to be overexpression of EGFR, which appears in more than 60% of triple negative breast cancer cases, the high malignancy of the tumor itself, and the tumor's metastatic rate; however, above all, there is no suitable target therapy due to the absence of the erbB-2 receptor or an estrogen hormone receptor in the luminal type of breast cancer.
Silibinin has been known to have several anticancer effects for some kinds of human cancer. Bhatia et al. [10] reported research findings that silibinin inhibits DNA synthesis, cell cycle, and cell differentiation in several types of cancer cells, including breast cancer, and that it promotes cell apoptosis. Kaur et al. [6] and Singh et al. [7] have also reported that, for colon cancer and prostate cancer, silibinin acts on different intracellular molecule signal systems to prevent cancer cell proliferation, and that it promotes apoptosis.
Based on the above-mentioned research findings on silibinin's anticancer effect for several types of cancer cells, we have studied the action mechanism for the anticancer effects of silibinin for MCF7 cells and SKBR3 cells, which are breast cancer cells, and MDA-MB-468 and MDA-MB-231, which are known to be triple negative breast cancer cells. In 2009, it was found that when 50 µg/mL of silibinin was used to treat the growth of MCF7 and MDA-MB-231 breast cancer cells induced by TPA (12-O-tetradecanoyle phorbol-13-acetate), expression of COX-2, VEGF, and MMP-9 was inhibited; it was also found that the mechanism for inhibiting the expression of COX-2, VEGF, and MMP-9 was achieved through inhibition of the Raf/MEK/ERK signal system [8,9]. That is, it was found that silibinin inhibits generation of COX-2, VEGF, and MMP-9, which cause infiltration and metastasis of breast cancer cells, by inhibiting the Raf/MEK/ERK downstream signal system. In addition, in 2010, by using MDA-MB-231 cell lines, which is one of the triple negative breast cancer cell lines, research confirmed the mechanism by which silibinin both induces arrest of the G2/M phase through down-regulation and cyclin B1 and cdc2 protein, and the up-regulation of p21 protein, and inhibits the growth of breast cancer cells [11]. Recently, the EGFR-inhibitory effect of silibinin was found by using SKBR3 cells; that study found that EGFR is overexpressed among breast cancer cells [12]. When EGF was treated in SKBR3 breast cancer in that experiment, the phosphorylation process of EGFR was induced and it was found that EGFR phosphorylation is inhibited in SKBR3 breast cancer cells when 50 µg/mL of silibinin was treated with EGF; moreover, inhibition of expression of MMP-9 protein and ERK expression, the final step of the Raf/MEK/ERK signal system, could be confirmed through Western blotting [12]. As shown above, through a variety of mechanisms, the antitumor effect of silibinin on breast cancer cells has been found through in vitro experiments and, based on these results, we conducted an experiment to see if silibinin showed the same antitumor effect in experiments using MDA-MB-468, another triple negative breast cancer cell line, that would be similar to the effect that silibinin has been shown to have on MDA-MB-231; we also aimed to see if it showed a similar antitumor effect in vitro in an MDA-MB-468 breast cancer cell implanted mouse animal model experiment.
Singh et al. [13] investigated the inhibitory effect of silibinin on bladder cancer in a study that compared a silibinin-treated group, which was administered 200 mg/kg of silibinin, and a control mice group that was not treated with silibinin, after transplanting RT4 bladder cancer cells in experimental mice; they reported that the size of the tumor was reduced by 58% in the group that was administered 200 mg/kg of silibinin for about 12 weeks as compared to the control mice (P < 0.001). In 2008, Singh et al. [7] also reported that the size of the tumor was reduced by 68% in the silibinin-treated mice compared to the control mice through the animal model of prostate cancer (P < 0.001). Moreover, in that experiment, they reported that the density of the microvessel around the tumor tissue was reduced by about 60% in the silibinin-treated mice (P < 0.001), and the VEGF and MMP that are known to promote angiogenesis and infiltration of tumor surrounding tissue were reduced in the silibinin-treated mice [7]. Based on the results of this experiment, Singh et al. [7] reported that silibinin can inhibit the growth of tumors as well as microvascular generation for infiltration and metastasis of cancer cells around the tumor. Chang et al. [14] reported that, in a renal cancer cell 786-O transplanted mouse animal model experiment, the tumor size of the group treated with 200 mg/kg of silibinin daily for 44 days was reduced by 70.1% compared to that of control mice that were not treated with silibinin (P < 0.001); in that experiment, if the 786-O cell was treated with silibinin, expression of MMP-2 and MMP-9 was inhibited. In this current experiment, it was found that when the triple negative breast cancer MDA-MB-468 cell line was transplanted into immunosuppressed female Balb/c-nude mice, and then 200 mg/kg of silibinin was orally administered for 45 days, the size of the tumor was reduced by 52.8% compared to the control mice that were not treated with silibinin (silibinin-treated mice vs. control mice; 230.3 ± 61.6 mm3 vs. 435.7 ± 93.5 mm3, P < 0.001) and the anticancer effect of silibinin on breast cancer cells shown in conventional in vitro experiments was also found in in vivo experiments using triple negative breast cancer cells. Moreover, when EGF was treated in MDA-MB-468 to find the inhibitory effect of silibinin on EGFR overexpressed in MDA-MB-468 cells, phosphorylation of EGFR increased in the control mice while phosphorylation of EGFR was inhibited in the mice treated with 50 µg/mL of silibinin. This finding was the same as the results of a previous research experiment that we conducted on ERK and MMP-9 generation inhibition by phosphorylation inhibition of EGFR of silibinin in SKBR3 breast cancer cells [8]. Finally, real-time PCR was conducted by using the tumor tissue of mice that had been engrafted with MDA-MB-468 breast cancer cells and, as a result, expression of COX-2, VEGF, and MMP-9 was inhibited up to 50%-80% compared to that of the control mice. The same experimental findings were shown in the results of Western blotting analysis. That is, it turned out that silibinin is a drug that is able to sequentially inhibit the subsignal systems by inhibiting the phosphorylation process of EGFR in vivo as well as in vitro, thereby, effectively blocking generation of VEGF, COX-2, and MMP-9 that are involved in growth, infiltration and metastasis of tumors. In conclusion, this experiment, which identified the anticancer effect of silibinin in mice engrafted with MDA-MB-468 triple negative breast cancer cells, is the first important experiment confirming that silibinin can show obvious growth inhibitory effects for breast cancer in vitro as well as in vivo through a triple negative breast cancer mouse model. In the future, we will conduct an experiment to see if the same result can be derived in a human breast cancer tissue transplanted animal model. Ultimately, we expect that silibinin can be used as a subsidiary medicine for triple negative breast cancer patients that have no specific treatment except for anticancer chemotherapy.
Figures and Tables
Fig. 2
Epidermal growth factor receptor (EGFR) family expression of MDA-MB-231 and MDA-MB-468 cell line using Western blotting.
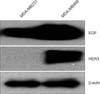
Fig. 3
Time-dependent change in tumor size of the control mice and the silibinin-treated mice. (A) Tumor size of the control mice and the silibinin-treated mice on the 45th day of silibinin administration; (B) tumor size of the control mice and the silibinin-treated mice extracted after mouse euthanasia; (C) difference of the mean tumor volume for each group on the 3rd day, the 10th day, the 17th day, the 24th day, the 31st day, the 38th day, and the 45th day for the silibinin-treated mice and the control mice. *P < 0.05; **P < 0.01.
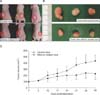
Fig. 4
Epidermal growth factor receptor (EGFR) phosphorylation of MDA-MB-468 breast cancer cell treated with silibinin using Western blotting. Con, control.

Fig. 5
VEGF, COX-2, and MMP-9 gene expression using tumor tissue extracted from the triple negative breast cancer animal model. (A) According to real-time polymerase chain reaction analysis for tumor tissue, COX-2, MMP-9, and VEGF gene expression was reduced by 50%-80% in the silibinin-treated mice compared to the control mice; (B) Western blotting analysis also showed that cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) protein expression was reduced in the silibinin-treated mice.

ACKNOWLEDGEMENTS
This experiment was supported by the fund of Samsung Biomedical Research Institute.
References
1. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–10874.
2. Reis-Filho JS, Natrajan R, Vatcheva R, Lambros MB, Marchio C, Mahler-Araujo B, et al. Is acinic cell carcinoma a variant of secretory carcinoma? A FISH study using ETV6 'split apart' probes. Histopathology. 2008; 52:840–846.
3. Mereish KA, Bunner DL, Ragland DR, Creasia DA. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: biochemistry, histopathology, and lethality. Pharm Res. 1991; 8:273–277.
4. Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006; 98:846–855.
5. Jiang C, Agarwal R, Lu J. Anti-angiogenic potential of a cancer chemopreventive flavonoid antioxidant, silymarin: inhibition of key attributes of vascular endothelial cells and angiogenic cytokine secretion by cancer epithelial cells. Biochem Biophys Res Commun. 2000; 276:371–378.
6. Kaur M, Velmurugan B, Tyagi A, Deep G, Katiyar S, Agarwal C, et al. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol Cancer Ther. 2009; 8:2366–2374.
7. Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008; 14:7773–7780.
8. Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009; 16:573–580.
9. Kim S, Kim SH, Hur SM, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents TPA-induced MMP-9 expression by down-regulation of COX-2 in human breast cancer cells. J Ethnopharmacol. 2009; 126:252–257.
10. Bhatia N, Zhao J, Wolf DM, Agarwal R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin. Cancer Lett. 1999; 147:77–84.
11. Kim S, Lee HS, Lee SK, Kim SH, Hur SM, Kim JS, et al. 12-O-Tetradecanoyl phorbol-13-acetate (TPA)-induced growth arrest is increased by silibinin by the down-regulation of cyclin B1 and cdc2 and the up-regulation of p21 expression in MDA-MB231 human breast cancer cells. Phytomedicine. 2010; 17:1127–1132.
12. Kim S, Han J, Kim JS, Kim JH, Choe JH, Yang JH, et al. Silibinin suppresses EGFR ligand-induced CD44 expression through inhibition of EGFR activity in breast cancer cells. Anticancer Res. 2011; 31:3767–3773.
13. Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin Cancer Res. 2008; 14:300–308.
14. Chang HR, Chen PN, Yang SF, Sun YS, Wu SW, Hung TW, et al. Silibinin inhibits the invasion and migration of renal carcinoma 786-O cells in vitro, inhibits the growth of xenografts in vivo and enhances chemosensitivity to 5-fluorouracil and paclitaxel. Mol Carcinog. 2011; 50:811–823.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download