Abstract
Purpose
Hepatic resection is a standard method of treatment for colorectal liver metastases (CRLM). However, the pathologic factors of metastatic lesions that affect tumor recurrence are less well defined in CRLM. The aim of this study was to evaluate the risk factors for recurrence of CRLM, focusing on histopathologic factors of metastatic lesions of the liver.
Methods
From January 2003 to December 2008, 117 patients underwent curative hepatic resection for CRLM were reviewed. Tumor size and number, differentiation, tumor budding, angio-invasion, dedifferentiation and tumor infiltrating inflammation of metastatic lesions were investigated.
Results
The mean number of hepatic tumors was 2 (range, 1-8). The mean size of the largest tumor was 2.9 cm (range, 0.3-18.5 cm) in diameter. The moderate differentiation of the hepatic tumor was the most common in 86.3% of the patients. Tumor budding, angio-invasion, and dedifferentiation were observed in 81%, 34%, and 12.8% of patients. Inflammation infiltrating tumor was detected in 6.8% of patients. Recurrence after hepatic resection appeared in 69 out of 117 cases (58.9%). Recurrence-free survival at 1, 2 and 5 years were 62.4%, 43.6%, and 34.3%. The multivariate analysis showed the number of metastases ≥3 (P = 0.007), the tumor infiltrating inflammation (P = 0.047), and presence of dedifferentiation (P = 0.020) to be independent risk factors for tumor recurrence.
Hepatic resection is a standard method of treatment for colorectal liver metastases (CRLM). Many articles have described the benefits of surgery for liver metastases of colorectal carcinoma (CRC), resulting in a 5-year survival of up to 50% [1,2,3,4]. Nevertheless, tumor recurrence after curative resection remains a major problem. Approximately, 50%-75% of patients develop recurrence of the disease after curative resection of CRLM [4,5].
Numerous studies have investigated the factor associated with recurrence after hepatectomy in CRLM. Several clinical factors such as preoperative CEA level, primary colorectal tumor stage, differentiation and lymph node (LN) metastasis of primary colorectal tumor, metastatic tumor burden, time interval to the metastasis, and neoadjuvant/adjuvant chemotherapy have been established as important determinants of tumor recurrence in CRLM [6,7,8]. However, early recurrence sometimes was developed in patients with known good prognostic factors. For this reason, there has been tumor biology suggestion to determine additional tumor aggressiveness [9].
Recently, several studies have been performed to analyze the histopathology of metastatic lesions as a predictive marker of tumor recurrence or a prognosis in patients with colorectal lung metastasis. Shirakusa et al. [10] suggested that the histologic appearance of metastatic lesion was associated with poor survival and higher LN metastasis in colorectal lung metastases. Shiono et al. [11] demonstrated the association with the pathologic factors of metastatic lesions such as aerogenous spread with floating cancer cell clusters and vascular invasion with prognosis in colorectal lung metastases. But the pathologic factors of metastatic liver lesions that affect tumor recurrence are less well defined in CRLM.
Therefore, the aim of this study was to clarify the characteristics and identify the risk factors for recurrence of the colorectal liver metastases, focusing on the histopathologic factors of metastatic hepatic lesions.
From January 2003 to December 2008, 226 patients underwent hepatic resection for CRLM. Among them, 117 patients who underwent curative resection and who underwent precise histologic evaluation of metastatic lesions were retrospectively reviewed.
The CRLMs were found by routine protocol of follow-up of CRC for metachronous CRLM, i.e., serum CEA and abdomen contrast enhanced CT per 6 months after surgery for primary CRC. The synchronous CRLM was initially detected by preoperative evaluation protocol of CRC, i.e., abdomen contrast enhanced CT and/or rectal MRI for rectal cancer. In the case of suspicion of CRLMs, enhanced dynamic liver MRI, enhanced chest CT, and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scans were performed.
Neoadjuvant chemotherapy was given in patients with high preoperative CEA levels or stage M1b, M1c according to European Colorectal Metastases Treatment Group [12]. All patients underwent adjuvant chemotherapy. Selection criteria for hepatic resection included the following: the primary disease must be controlled, preoperative imaging studies revealed no extrahepatic disease except selected patients with controlled extrahepatic lesions, a hepatic resection would remove all tumors with acceptable surgical margins, and an expected adequate liver function after hepatic resection.
The resected specimens were immediately fixed in 10% buffered formalin and the tumors were cut into step wise sections and embedded in paraffin and stained with hematoxylin and eosin. The tumor size, number, major differentiation, angio-invasion, tumor budding, dedifferentiation and tumor infiltrating inflammation grade were investigated by a single pathologist who is an expert in liver pathology (K.B.L.). Angio-invasion was defined as the presence of cancer cells within endothelial-lined channels. An isolated cell or a small cluster of <5 carcinoma cells in the invasive front was defined as a budding focus, and ≥10 budding foci when viewed at a 200-fold magnification was considered positive for tumor budding. Dedifferentiation was defined as microscopic clusters of tumor cells with large solid sheet, cribriform pattern, spindle cell morphology, trabecular or palisading pattern or extensive single cells (more than 50% of tumor). Tumor infiltrating inflammation was defined as the presence of tumor infiltrating lymphocytes, neutrophils, or macrophages.
Patients were examined for CRLM recurrence by contrast enhanced CT every 4-6 months and blood test including tumor marker of CEA, every 2-3 months after discharge. When hepatic recurrence was suspected, dynamic enhanced liver MRI was performed to ensure the appearance of new lesions in the remnant liver, while systemic recurrence was examined by 18F-FDG PET. Recurrence was diagnosed when at least two imaging studies confirmed the new lesions showing typical features of CRC/CRLM, compared tothe previous images.
Data are presented as mean ± standard deviation. Kaplan-Meier estimates of recurrence-free survival and recurrence was calculated and compared with the log-rank test. Multivariate Cox modeling was performed using potential risk factors (demographic factors: gender, age, preoperative CEA level, onset of liver metastasis, neo-adjuvant chemotherapy, presence of extrahepatic lesion; primary tumor factors: location, T stage, LN metastasis, histologic type; metastatic tumor factors: resection type, resection margin, number, largest size of tumor, major differentiation, budding, angio-invasion, dedifferentiation, tumor infiltrating inflammation). Forward stepwise selection using likelihood ratios for entry and exit criteria were used to develop the final multivariate Cox proportional hazards model. A P-value of <0.05 was considered statistically significant.
Two hundred eighty nine patients underwent hepatic resection for CRLM. After excluding 109 patients (37.7%) without the precise pathologic evaluation of metastatic lesion or with microscopic remaining tumors, 117 patients were enrolled in this study and evaluated. The patients consisted of 73 men (62.4%) and 44 women (37.6%) with a mean age of 64 years. Mean preoperative CEA level was 47.5 ± 16.7 ng/mL (range, 1-1,660 ng/mL). Synchronous liver metastasis was noted in 78 patients (66.7%). The majority of the primary tumor stage was T3 (n = 91, 77.8%). Metastases to a regional LN of primary CRC were noted in 82 patients (71.3%). Moderate differentiation occupied the most of differentiation (n = 90, 86.5%) (Table 1).
The mean number of hepatic tumors was 1.7 ± 0.1 (range, 1-8). The mean size of the largest tumor was 2.9 ± 0.2 cm (range, 0.3-18.5 cm) in diameter. Moderate differentiation occupied the most of differentiation (n = 101, 86.3%). Tumor budding was observed in 81.2% (n = 95) and angio-invasion was observed in 34.2% of patients (n = 40). Dedifferentiations were observed in 12.8% of patients (n = 15, Fig. 1); large solid sheet (n = 7, Fig. 1A), palisading pattern (n = 4, Fig. 1B), cribriform pattern (n = 2, Fig. 1C), extensive single cells (n = 2, Fig. 1D). Mild to moderate inflammation infiltrating tumor was detected in 6.8% of patients (n = 8, Fig. 2) (Table 2).
Median follow-up was 34.2 months. Overall survival at 1, 2, and 5 years were 95.7%, 93.7%, and 67.5% (Fig. 3). In multivariate analysis, old age (>60 years) (hazard ratio [HR], 9.160; 95% confidence interval [CI], 1.190-70.485, P = 0.033) and number of metastases (≥3) (HR, 3.283; 95% CI, 1.222-8.820, P = 0.018) were associated with poor patient survival.
Recurrence of CRC after hepatic resection appeared in 69 cases. Recurrence-free survival at 1, 2, and 5 years were 62.4%, 43.6%, and 34.3% (Fig. 4). The extrahepatic recurrence was noted in 48.5%. However, the most frequent site of initial recurrence was the liver in 54 patients (41.5%), and then a metastatic LN in 32 (24.6%), the lung in 24 (18.5%), and peritoneal seeding in 7 (5.4%). Multiple site recurrence was also noted in 13 (10.0%). The multivariate analysis showed the number of metastases (≥3) (HR, 2.241; 95% CI, 1.251-4.014, P = 0.007), the inflammation infiltrating tumor (HR, 2.388; 95% CI, 1.012-5.634, P = 0.047), and presence of histologic dedifferentiation (HR, 2.152; 95% CI, 1.129-4.102, P = 0.020) to be independent risk factors for tumor recurrence after hepatic resection in CRLM (Table 3).
The positive histopathologic markers predicting tumor recurrence, i.e., dedifferentiation or tumor infiltrating inflammation, were observed in 22 patients. Between patients with predictive histopathologic markers of recurrence and those without those, there was no statistically difference in preoperative CEA level, metastases to a regional LN of primary CRC, the mean number of tumor and the mean size of the largest tumor (Table 4). The recurrence free survival rates of those patients with predictive histopathologic markers were 50.0% at 1 year and 22.7% at 2 years after hepatic resection. It seemed to be poorer than others whose recurrence free survival rates were 65.2% at 1 year and 48.9% at 2 years. However, there was no statistical significance (P = 0.062). Baseline demographics and tumor characteristics are shown in Table 5. Although 13 patients (No. 1-13) had more than one of the well-known good prognostic factors, i.e., preoperative CEA level <200 ng/mL, and size of metastases ≤3 cm, as well as the number of metastasis less 3, they showed recurrence of the tumor within 2 years after hepatic resection.
Despite recent improvements in the diagnosis and treatment of CRLM, the long-term survival of patients is unsatisfactory because of tumor recurrence after hepatic resection. Therefore, it is necessary to find predictive factors relevant to tumor recurrence to improve the survival outcomes.
Several studies showed that histopathologic factors of primary CRC were related to liver metastasis. Inomata et al. [13] reported the correlation between CRLM and dedifferentiation, angio-invasion and tumor inflammatory cell infiltration of primary CRC. Ono et al. [14] demonstrated that focal dedifferentiation at the invasive front of primary CRC was associated with liver metastases. However, most previous studies about factors associated with recurrence focused on histopathologic examination of primary CRC and there had been a few reports on the histologic appearance of metastatic lesions in CRLM.
Therefore, we hypothesized that histopathologic factors of metastatic lesions such as dedifferentiation, angio-invasion, tumor infiltrating inflammation will be associated with tumor recurrence after hepatic resection in CRLM. The results of our study revealed that the tumor infiltrating inflammation and presence of dedifferentiation of metastatic lesion were independent risk factors for tumor recurrence after hepatic resection in CRLM.
Dedifferentiation is microscopic clusters of undifferentiated cancer cells or single carcinoma cells or a solitary trabecular form. Dedifferentiation exhibits a desmoplastic stromal reaction with marked fibrosis and neovascularization at the tumor invasive front. Tominaga et al. [15] reported that a high grade of dedifferentiation is an important predictor of LN metastasis in patients with nonpedunculated submucosal invasive CRC. Inomata et al. [13] demonstrated the association of dedifferentiation with liver metastasis in CRC. Our results showed that the presence of dedifferentiation had a significant influence on tumor recurrence (P = 0.020). Therefore, it is necessary to evaluate the presence of dedifferentiation in metastatic lesions to predict tumor recurrence.
Tumor infiltrating inflammation is considered to be pathological characteristics corresponding to host antitumor response and has been reported to be associated with metastatic activity and prognostic outcome. Unitt et al. [16] demonstrated that the tumor lymphocytic infiltrate is associated with hepatocellular carcinoma recurrence after liver transplantation. Ding et al. [17] showed that high macrophage infiltration predicts high recurrence and poor survival in patients with hepatocellular carcinoma. Similarly, the mild to moderate tumor infiltrating inflammation was associated with high recurrence in our current study.
Dedifferentiation and tumor infiltrating inflammation of the metastatic lesion could be one of the risk factors of aggressive behavior as well as size and number of metastases. In this study, although 13 patients had good prognostic factors, as noted in the results session, aggressive tumor biology like dedifferentiation or tumor infiltrating inflammation deviated the outcome; 6 patients developed tumor recurrence within 6 months and the additional 3 patients showed recurrence within 12 months after hepatic resection in CRLM. The recurrence free survival rates of these patients seemed to be lower than the others although there was no statistical significance. This might be a type II error associated with small sample size.
In our study, there is no significant difference in histopathology according to neoadjuvant and adjuvant chemotherapy, although this study has the limitations of retrospective design and small sample size of the neoadjuvant chemotherapy group. However, for this reason, it was possible to analyze the exact histopathological changes of the metastatic tumors.
In this study, we identified the pathologic prognostic markers of hepatic tumors predicting prognosis of the patients with CRLM although they had undergone curative hepatic resection. Dedifferentiation and tumor infiltrating inflammation of metastatic lesions were predictive factors of tumor recurrence in CRLM. Our results might give a clinical guideline to determine use of adjuvant chemotherapy after curative resection of CRLM. However, because the study design was a retrospective review for the small number of patients, further validation is required.
Figures and Tables
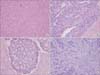 | Fig. 1Histology of dedifferentiation. Dedifferentiation was defined as microscopic clusters of tumor cells with large solid sheet (A), palisading pattern (B), cribriform pattern (C), extensive single cells (D) (A-D: H&E, ×200). |
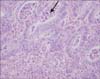 | Fig. 2Tumor infiltrating inflammation was defined as the presence of tumor infiltrating lymphocytes, neutrophils, or macrophages (arrow) (H&E, ×400). |
 | Fig. 4Recurrence free survival of the 117 patients after hepatic resection for colorectal liver metastases. |
Table 1
Baseline and primary tumor characteristics of 117 patients with CRLM who underwent hepatic resection

Table 2
Liver metastatistic tumor characteristics of 117 patients with CRLM who underwent hepatic resection

References
1. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002; 235:759–766.
2. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007; 25:4575–4580.
3. Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Sakamoto M, Fukuda H. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br J Surg. 1999; 86:332–337.
4. Yokoi Y, Suzuki S, Nakamura S. The impact of hepatic resection on metastatic colorectal cancer. Gan To Kagaku Ryoho. 2002; 29:848–855.
5. Malafosse R, Penna C, Sa Cunha A, Nordlinger B. Surgical management of hepatic metastases from colorectal malignancies. Ann Oncol. 2001; 12:887–894.
6. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008; 371:1007–1016.
7. Sorbye H, Mauer M, Gruenberger T, Glimelius B, Poston GJ, Schlag PM, et al. Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann Surg. 2012; 255:534–539.
8. Son SY, Yi NJ, Hong G, Kim H, Park MS, Choi YR, et al. Is neoadjuvant chemotherapy necessary for patients with initially resectable colorectal liver metastases in the era of effective chemotherapy. Korean J Hepatobiliary Pancreat Surg. 2011; 15:206–217.
9. Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Interaction of tumour biology and tumour burden in determining outcome after hepatic resection for colorectal metastases. HPB (Oxford). 2010; 12:84–93.
10. Shirakusa T, Tsutsui M, Motonaga R, Ando K, Kusano T. Resection of metastatic lung tumor: the evaluation of histologic appearance in the lung. Am Surg. 1988; 54:655–658.
11. Shiono S, Ishii G, Nagai K, Yoshida J, Nishimura M, Murata Y, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg. 2005; 79:278–282.
12. Nordlinger B, Van Cutsem E, Rougier P, Köhne CH, Ychou M, Sobrero A, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007; 43:2037–2045.
13. Inomata M, Ochiai A, Sugihara K, Moriya Y, Yamaguchi N, Adachi Y, et al. Macroscopic features at the deepest site of tumor penetration predicting liver metastases of colorectal cancer. Jpn J Clin Oncol. 1998; 28:123–128.
14. Ono M, Sakamoto M, Ino Y, Moriya Y, Sugihara K, Muto T, et al. Cancer cell morphology at the invasive front and expression of cell adhesion-related carbohydrate in the primary lesion of patients with colorectal carcinoma with liver metastasis. Cancer. 1996; 78:1179–1186.
15. Tominaga K, Nakanishi Y, Nimura S, Yoshimura K, Sakai Y, Shimoda T. Predictive histopathologic factors for lymph node metastasis in patients with nonpedunculated submucosal invasive colorectal carcinoma. Dis Colon Rectum. 2005; 48:92–100.
16. Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006; 45:246–253.
17. Ding T, Xu J, Wang F, Shi M, Zhang Y, Li SP, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009; 40:381–389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download