Abstract
Peritoneal dissemination is one of the treatment failures following gastric cancer surgery. We present a case with very early peritoneal recurrence, detected 8 days following curative surgery. A 39-year-old man, with Borrmann-4 advanced gastric cancer with signet ring cell type, underwent curative open total gastrectomy. However, focal peritoneal nodules on the left side of the diaphragmatic surface, which did not exist at the initial operation, were incidentally found during the reoperation for a postoperative intestinal obstruction via a laparoscopic approach. The pathologic result of the biopsied nodule revealed signet ring cell carcinoma. The patient underwent combination chemotherapy for several months without tumor regression. He suffered from intestinal obstruction again due to carcinomatosis peritonei, and died 9 months following initial surgery. Through this case report, we can carefully suspect that very early progression of cancer cells to carcinomatosis can occur in just several days after an operation.
Gastric cancer can spread to other organs through hematogenous and lymphatic routes or direct peritoneal dissemination. In particular, peritoneal dissemination is quite frequent in patients with gastric cancer and presumably originates from the free cancer cells exfoliated from the cancer-penetrated serosal layer [1]. Generally, young age, infiltrative or diffuse type, and undifferentiated histologic subtypes are poor prognostic factors regarding recurrence following curative resection (R0 resection) for advanced gastric cancer (AGC) [2,3]. Postoperative recurrence is usually found within 1-2 years after curative resection for AGC [3]. In cases of early recurrence within 2 months after resection, there is high probability of inadequate R0 resection such as the existence of a systemic hidden metastasis at the time of operation or free cancer cells released from gastric lumen or lymphovascular channels during radical gastric cancer surgery. Nevertheless, very early recurrence, 8 days after curative R0 resection with open total gastrectomy with D2 lymph node dissection for AGC, is extremely rare.
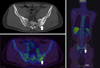
A 39-year-old man with progressive weight loss of 10 kg during the past 6 months was referred to Departement of Surgery in June 2012. No definite abnormal findings in the stomach or duodenum were observed on gastrofiberscopy (GFS) 1 year ago at Yeouido St. Mary's Hospital (Fig. 1A). However, diffuse engorged gastric folds with ulcerations at the mid body and greater curvature side of the stomach, suggesting a Borrmann-4 AGC were found during the current GFS (Fig. 1B). A biopsy confirmed signet ring cell carcinoma. An abdominal computed tomography (CT) scan also showed diffuse gastric wall thickening, compatible with the GFS findings without definite regional lymphadenopathy (cT4aN0M0) (Fig. 2). Positron emission tomography (PET) CT found no active lesions in the abdomen including the stomach and liver. However, a mild hypermetabolic focus at the medial aspect of the left iliac bone was noted, which was equivocal to confirm the bone metastasis (Fig. 3). Tumor markers were all within normal limits including carcinoembryonic antigen of 0.6 ng/mL (normal range, 0-5 ng/mL), alpha fetoprotein of 1.6 ng/mL (normal range, <8.1 ng/mL) and carbohydrate antigen 19-9 of 6.68 U/mL (normal range, 0-37 U/mL).
An open total gastrectomy and splenectomy with D2 lymph node dissection was carried out for the R0 resection. No definite metastatic focus was detected during the operation, including definite metastatic lymphadenopathy in the entire abdominal cavity. No peritoneal washing cytology was performed. The postoperative course was uneventful until postoperative day 4 when he resumed soft meals.
A pathological examination revealed Borrmann-4, 13.0 cm × 11.0 cm sized serosa-exposed signet ring cell carcinoma with 50 metastatic lymph nodes out of 84 retrieved lymph nodes resulting in pT4aN3bM0, stage IIIc according to Union of International Cancer Control seventh edition. There was no microscopic cancer cell involvement on either resection margin, resulting in a R0 resection.
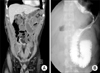
Five days following the gastrectomy, localized abdominal pain on the left upper quadrant with nausea and vomiting developed. A 500-mL volume of turbid whitish fluid without bile contents were aspirated via a reinserted Levin tube. An abdominal CT scan and Gastrografin swallowing radiography showed a markedly dilated jejunal Roux-limb with an abrupt cutoff near the jejuno-jejunostomy site (Fig. 4). All attempts to improve the intestinal obstruction, including nasojejunal decompression and correction of the fluid and electrolyte imbalance were unsuccessful. A re-exploration was considered to relieve the intestinal obstruction.
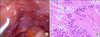
A reoperation to relief the Roux-limb obstruction via a laparoscopic approach was performed at postoperative day 8. Adhesions were observed around the jejunojeunostomy site and the mesocolon compressed the Roux-limb just proximal to the jejunojejunostomy. Laparoscopic adhesiolysis relieved the Roux-limb obstruction without any problem. However, we incidentally found some whitish peritoneal nodules on the left diaphragmatic surface that were not present at the initial operation (Fig. 5A). One of peritoneal nodules was excised for histologic examination, revealing a metastatic adenocarcinoma with signet ring cell features (Fig. 5B). The patient tolerated the reoperation well and was transferred to the Department of Internal Medicine for systemic combination chemotherapy of capecitabine and cisplatin (XP).
During the follow-up examination performed in December 2012, 6 months after the operation and after the eight cycle of XP chemotherapy, a CT scan showed newly developed ill-defined soft tissue density in the common hepatic space and retropancreatic space, suggesting nonspecific postoperative changes or a tumor recurrence. Then, the chemotherapy regimen was changed to oxaliplatin-based FOLFOX. In addition, no interval change in the 1.7 cm sized sclerotic bony lesion in the left iliac bone was observed on a serial bone and CT scan.
The patient tolerated a regular diet and chemotherapy relatively well until January 2013. Then, abrupt vomiting developed. A CT scan and an upper gastrointestinal series showed markedly distended jejunal limb due to cancer recurrence. Bypass jejunojejunostomy was performed to relieve the jejunal obstruction at the jejunojejunosotmy site. Marked carcinomatosis and ascites were found during the operation. After the jejunojeunal bypass, the symptoms were relieved temporarily, but the patient suffered from poor oral intake and a large volume of ascites. The patient died on March 9, 2013. He had survived 9 months following the initial operation.
Radical gastrectomy with D2 lymph node dissection is the principle treatment for AGC according to the Japanese gastric cancer treatment guidelines [4]. However, many patients with AGC suffer from tumor recurrence following curative radical gastrectomy, even if no residual tumor remains after surgical resection (R0 resection).
Peritoneal recurrence represents one of the most common causes of failure after curative surgery for gastric cancer, constituting 32%-54% of recurrences and occurs through a number of mechanisms [5]. Cancer cells spread across the peritoneal cavity when they penetrate the full thickness of the gastric wall (T4a). In addition, they can be released from lymphovascular pedicles or iatrogenic inappropriate tumor handling at the time of surgery [6].
The incidence of peritoneal recurrence varies in relation to different clinicopathological factors. Diffuse-mixed histological type, serosa invasiveness, lymph node status, and diameter of the tumor have been identified as independent predictors of peritoneal recurrence. Yoo et al. [2] reported that peritoneal recurrence is associated with younger age, infiltrative or diffuse type, an undifferentiated tumor, and total gastrectomy; older age and larger tumor for disseminated hematogenous recurrence; and older age, large tumor size, infiltrative or diffuse type, proximal tumor and subtotal gastrectomy for locoregional recurrence. In addition, postoperative mean length of time to recurrence was 21.8 months for overall recurrence and 18.1 months for peritoneal recurrence.
Cytologic evidence of free cancer cells in the peritoneum is considered an indicator of a poor prognosis in patients with gastric cancer [1,7]. At the time of operation, peritoneal washing cytology to detect free cancer cells plays an important role predicting peritoneal dissemination. According to the recommendations of the Japanese Classification of Gastric Carcinoma, positive washing cytology is a poor prognostic factor, and the results of cytology are incorporated into the stage classification as the CY category, in which CY1 stands for positive cytology and classifies the patients into stage IV [8]. In our case, we did not perform peritoneal washing cytology, which could be a limitation of our case to identify the origin of the peritoneal seeding, pre-existing free cancer cells, or spilled cancer cells during the operation. We should try to find the possible peritoneal dissemination in the operation of far AGC carefully including cytology not to miss the peritoneal dissemination.
Positive washing cytology is known as poor prognostic factor for peritoneal metastasis and survival. However, the natural course of peritoneal metastasis has not been fully demonstrated, and immediate progression of peritoneal seeding has not been reported. If an intestinal obstruction had not developed, this patient would have undergone adjuvant chemotherapy, and then progression and regression would be repeated during the period of adjuvant chemotherapy. Then, peritoneal seeding would have been detected at some point during the follow up examination, as in other patients. Although the peritoneal recurrence is usually found within 1-2 years after curative resection for far AGC, the starting time of recurrence is probably faster than we thought. However, we don't know the exact time of the recurrence. This case lets us know that recurrence can be very rapidly developed.
Occasionally, a postoperative CT scan demonstrates a small amount of ascites with peritoneal thickening within a few days after curative gastric resection for AGC which is difficult to distinguish from postoperative changes. Most surgeons seem to disagree that this represents a very early peritoneal recurrence because they believe that R0 resection was achieved. However, the results of our case suggest the possibility of very early peritoneal dissemination of cancer cells within a few days after R0 resection, particularly in patients with poor prognostic factors.
In this case, very early peritoneal seeding was incidentally detected during the surgical exploration for the intestinal obstruction not related with peritoneal seeding directly. However, 6 months later, the patient underwent bypass surgery for small bowel obstruction due to carcinomatosis. Intestinal obstruction can be an important sign of peritoneal seeding in far AGC patients.
Through this case report, we carefully suspect that very early progression of cancer cells to carcinomatosis can occur just after an operation, particularly in cases with poor prognostic factors, such as Borrmann-4, young age and undifferentiated tumor cell type. For these reasons, we should think the early peritoneal dissemination or other recurrence in the case of far AGC. Also, prompt initiation of systemic chemotherapy is necessary in these patients.
Figures and Tables
Fig. 1
Gastrofiberoscopic findings showed abrupt change in a year. (A) Normal gastric mucosal fold at midbody greater curvature side 1 year ago, (B) Diffuse thickening of the gastric mucosal folds and cent ral ulceration.
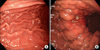
Fig. 2
Computed tomography shows diffuse gastric wall thickening suggesting Borrmann-4 gastric cancer.
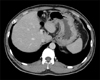
Fig. 3
Positron emission tomography-computed tomography shows equivocal hypermetabolic lesion at the sacroiliac joint (arrow).
References
1. Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999; 72:60–64.
2. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000; 87:236–242.
3. Otsuji E, Kuriu Y, Ichikawa D, Okamoto K, Ochiai T, Hagiwara A, et al. Time to death and pattern of death in recurrence following curative resection of gastric carcinoma: analysis based on depth of invasion. World J Surg. 2004; 28:866–869.
4. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.
5. Marutsuka T, Shimada S, Shiomori K, Hayashi N, Yagi Y, Yamane T, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003; 9:678–685.
6. Han TS, Kong SH, Lee HJ, Ahn HS, Hur K, Yu J, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol. 2011; 18:2818–2825.
7. Homma Y, Ushida S, Yamada M, Kobayashi H, Suzuki K. Positive peritoneal washing cytology in multiple cavities can predict poor prognosis of advanced gastric cancer patients. Ann Surg Oncol. 2010; 17:455–460.
8. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download