Abstract
Acute portal vein and mesenteric vein thrombosis (PVMVT) can cause acute mesenteric ischemia and be fatal with mortality rate of 37%-76%. Therefore, early diagnosis and prompt venous revascularization are warranted in patients with acute symptomatic PVMVT. Due to advances in catheter-directed treatment, endovascular treatment has been used for revascularization of affected vessels in PVMVT. We report two cases of symptomatic PVMVT treated successfully by transhepatic percutaneous mechanical thrombectomy-assisted thrombolysis.
Acute portal vein and mesenteric vein thrombosis (PVMVT) is a rare and insidious disease that is associated with significant mortality and morbidity. Because its clinical manifestations are almost asymptomatic or nonspecific, diagnosis is often delayed, which can have lethal consequences. Therefore, early diagnosis and prompt venous revascularization are critically important to effectively treat acute symptomatic PVMVT. Due to recent advances in catheter-directed treatment, an endovascular approach has been adopted by several groups as an alternative method to revascularize affected vessels in PVMVT, and several case studies have reported success using this method to treat PVMVT [1,2,3,4,5,6]. We report here two patients with PVMVT who were successfully treated by transhepatic percutaneous mechanical thrombectomy-assisted thrombolysis, while simultaneously avoiding unnecessary bowel resection.
A 40-year-old female presented to the Emergency Department with a 10-day history of vague abdominal pain, which had gradually aggravated and become intolerable. She had a 14-year drug history of oral contraceptives use, without any other medical history. Blood pressure was 120/80 mmHg, heart rate was 90 beats per minute, and body temperature was 37℃. On physical examination, there was abdominal tenderness and rebound tenderness in all quadrants, with decreased bowel sound. Initial laboratory study revealed white blood cell count, 11,000/µL (neutrophil, 86.8%); ESR, 27 mm/hr; CRP, 8.03 mg/L. An abdominal CT scan showed extensive thrombosis involving the portal vein and superior and inferior mesenteric veins with jejunal loop dilation and diffuse wall thickening combined with a small amount of ascites (Fig. 1). Mesenteric ischemia was suspected despite the patient's relatively mild clinical symptoms and stable vital signs; we therefore decided to perform percutaneous mechanical thrombectomy via a transhepatic approach rather than exploration.
Under ultrasound guidance, the portal vein was punctured with a micropuncture set (Cook Medical, Bloomington, IN, USA) and a 8-F guiding sheath was advanced to the right portal vein. A Terumo wire (Terumo Inc., Tokyo, Japan) was then introduced into the splenic vein and a 5-F multiple hole infusion Cragg-McNamara catheter (Covidien, Mansfield, MA, USA) was advanced over the wire. Contrast medium was injected into the splenic vein, which revealed extensive thrombosis in the portal vein, superior mesenteric vein, and splenic vein (Fig. 2A). Using an AngioJet Spiroflex thrombectomy catheter (Medrad International, Minneapolis, MN, USA), we performed mechanical thrombectomy of the splenic vein and superior mesenteric vein, and then pulled the catheter back into the main portal vein (Fig. 2B). Despite significant reduction of thrombus burden, venous flow was not restored sufficiently. We therefore inserted a multiple hole infusion catheter along the superior mesenteric vein (SMV), and performed thrombolysis with a high dose of urokinase (100,000 units/hr) for 24 hours. After 24-hour pharmacologic thrombolysis, the patient's abdominal pain had improved and her white blood cell count had returned to normal. Thrombolysis with urokinase was continued for an additional 24 hours until venous flow was restored in the follow-up portogram (Fig. 2C). After removal of the infusion catheter, the puncture site of the liver was coil embolized to prevent subcapsular bleeding (Fig. 2D, arrow). In the follow-up abdominal CT scan, the portal vein (PV) and SMV were well opacified with a small amount of remnant thrombus (Fig. 2D, E). Warfarin was used for anticoagulation with a target international normalized ratio range of 2.5 to 3.5. The patient was discharged on day 25 of her hospital stay. She is no longer taking oral contraceptives.
A 29-year-old male presented to the Emergency Department with a 7-day history of epigastric pain, which had recently aggravated. He had visited the local clinic 3 days prior, and had undergone a gastroduodenoscopy that had demonstrated hemorrhagic gastritis. He had no other medical history. Blood pressure was 136/90 mmHg with a heart rate of 102 beats per minute and a body temperature of 37.9℃. On physical examination, epigastric tenderness with diminished bowel sound was detected without signs of acute abdomen. Complete blood cell counts, liver function tests, and blood chemistry results were unremarkable, and ESR was 76 mm/hr, CRP, 14.88 mg/L; and D-dimer, 8.32 mg/L. The abdominal CT scan showed extensive thrombosis involving the portal vein, superior mesenteric vein, and splenic vein with cavernous transformation around the portal vein (Fig. 3A-C), and streaky infiltrations in the mesentery, raising the suspicion of mesenteric edema (Fig. 3C, D). A test for hypercoagulable states, including protein C and S activity, homocysteine, factor V Leiden mutation, the prothrombin 20210 mutation, fibrinogen, lupus anticoagulant antibody, and anticardiolipin antibody did not reveal any abnormalities except that fibrinogen was increased to 500 mg/dL. There were no clinical signs or radiographic images consistent with bowel ischemia, and the patient was therefore referred to the interventional radiology department for mechanical thrombectomy.
Under ultrasound guidance, the right portal vein was punctured with a 21 G Chiba needle (Cook Medical, Bloomington, IN, USA) and 018" Hair Wire (A&A Medical Device Co., Seongnam, Korea), and a 6-F Shuttle Flexor introducer sheath (Cook Medical) was advanced from the PV to the splenic vein and SMV. The venogram demonstrated extensive thrombosis in the portal vein, superior mesenteric vein, and splenic vein (Fig. 4A). After the venogram, aspiration thrombectomy was performed several times using a Luer-Lok syringe, and a small amount of thrombus was removed (Fig. 5). The follow-up venogram demonstrated only partial recanalization of the portal and superior mesenteric veins with sluggish flow. A 6-F AngioJet Solvent Omni catheter (Medrad International) was advanced over the guidewire and positioned in the distal SMV and splenic vein, and mechanical thrombectomy of the thrombosed segments of the splenic and superior mesenteric veins was performed (Fig. 4B). The catheter was then pulled back into the main portal vein. Despite a significant reduction in thrombus burden, venous flow was not restored sufficiently, so a 5-F multiside port infusion catheter (Cook Medical) was placed along the SMV, and thrombolysis was done with a high dose of urokinase (100,000 units/hr) for the initial 48 hours with intravenous heparin infusion (1,000 units/hr). Two days after the procedure, venography of the superior mesenteric and portal veins demonstrated partial recanalization of these vessels (Fig. 4C), and the patient's symptoms had also improved. After removal of the infusion catheter from the liver, the transhepatic tract was embolized with coils (Cook Medical) to reduce the risk of bleeding. In the follow-up abdominal CT scan, the large amount of thrombus in the PV and SMV had decreased with partial recanalization; however, a small amount of thrombus still remained in the PV, SMV, and splenic vein. Diet was started on postprocedure day 3, and anticoagulation therapy was switched from heparin to warfarin, with the target international normalized ratio range of 2.5-3.5. The patient was discharged at 16th hospital day.
Clinical manifestations of acute PVMVT are often asymptomatic or nonspecific (e.g., nausea, vomiting, anorexia, weight loss, diarrhea, and abdominal distension); diagnosis of PVMVT is therefore often delayed, which can be fatal. As with any venous thrombotic condition, the etiology of acute PV thrombosis can be categorized based on Virchow's triad of venous stasis (e.g., liver cirrhosis, Budd-Chiari syndrome, hypersplenism, obesity, etc.), hypercoagulable state (e.g., factor V leiden mutation, factor II G20210 mutation, protein C, S deficiency, malignancy oral contraceptive etc.), and endothelial injury (e.g., trauma, operation etc.) [1,3,4,5,6,7]. In this study, the first patient has a long-term history of oral contraceptive use; the seconpatient, however, had no identified risk factors. The availability of noninvasive imaging methods such as contrast enhanced CT, which can be used to diagnose PVMVT with a sensitivity of 90%, has facilitated early diagnosis and treatment [4]. Acute PVMVT has traditionally been treated by systemic anticoagulation therapy using heparin and warfarin to prevent propagation of the thrombus and to recanalize the vessels [1,3,4,5]. Surgery is warranted if patients demonstrate symptoms and signs of peritonitis. While medical and surgical treatment options are of limited utility in massive PVMVT, endovascular treatment is a therapeutic alternative that is minimally invasive and effective at thrombus removal or dissolution [1,3,4,5,6].
Percutaneous mechanical thrombectomy was first introduced over 20 years ago. It refers to the percutaneous removal of thrombus from arteries, veins, or vascular grafts without surgery, which is achieved by thrombus dissolution, fragmentation, aspiration of the thrombus, or combinations of these methods [2,3,4,7,8,9,10]. Two categories can be recognized: (1) percutaneous aspiration thrombectomy, where the thrombus is removed by steady manual suction through a large-lumen aspiration catheter used through a vascular sheath, and (2) mechanical thrombectomy, where the thrombus is macerated or fragmented in situ and removed with the use of specialized devices [7,8,9,10]. Mechanical thrombectomy has the advantages of rapid thrombus removal and shortening of the duration of thrombolytic infusion, thereby reducing potential bleeding complications [3,4,5]. Various mechanical thrombectomy devices are available and can be broadly divided into two categories: (1) hydrodynamic recirculation devices, which rely on the 'Venturi effect' generated by a high-speed retrograde saline jet (e.g., Hydrolyser device, Oasis thrombectomy system, AngioJet rheolytic thrombectomy device); and (2) rotational recirculation devices, which fragment the clot by producing a hydraulic vortex via a high-speed rotating impeller or basket (e.g., Amplantz thrombectomy device, Arrow-Trerotola device) [5,7,8,9,10]. We use an AngioJet thrombectomy catheter (Medrad International). This catheter generates a high-speed retrograde fluid jet at the tip that creates a high shear gradient through the Venturi effect, which fragments the thrombus. Debris is promptly evacuated through the lumen of the catheter [5,7,8,9,10]. Potential drawbacks of mechanical thrombectomy include increased risk of vessel injury or distal embolization, and high device cost.
Because PVMVT is a rare disease, the efficacy of endovascular intervention at treating PVMVT requires further evaluation, although there are some reports of combined percutaneous mechanical thrombectomy and pharmacological thrombolysis in patients with symptomatic PVMVT without surgical treatment. A search of the English medical literature in the PubMed database revealed 11 cases of symptomatic PVMVT patients who were treated with mechanical thrombectomy-assisted thrombolysis without surgical treatment (Table 1) [2,3,4,6]. Rosen and Sheiman [6] reported one case of symptomatic PV and SMV thrombus; the patient was successfully treated with mechanical thrombectomy using an AngioJet catheter and adjuvant infusion of plasminogen activator (tPA) via the superior mesenteric artery. Lopera et al. [2] reported two patients with symptomatic PV and SMV thrombosis who were successfully treated with an endovascular technique. Both patients underwent transhepatic percutaneous mechanical thrombectomy, one using an Oasis device and the other Arrow-Trerotola device, without any complications. Zhou et al. [3] reported two symptomatic PV and SMV thrombosis patients who were successfully treated by transhepatic percutaneous mechanical thrombectomy (AngioJet Rheolytic catheter) and pharmacologic thrombolysis. Kim et al. [4] reviewed 11 SMV thrombosis patients treated with percutaneous transhepatic catheter-directed thrombectomy and thrombolysis, and reported that this appears to be an effective approach for improving acute symptoms and long-term prognosis with a low complication rate. Seven of these patients underwent mechanical thrombectomy using an AngioJet device (five patients) or Helix clot Buster device (two patients); six of these patients were successfully treated while one patient who initially presented with SMV thrombosis in the setting of bacterial peritonitis and sepsis died of sepsis and multiple organ failure. These authors concluded that percutaneous mechanical thrombectomy/thrombolysis may lower the incidence of long-term morbidity and mortality. We treated two patients with PVMVT by percutaneous mechanical thrombectomy with an AngioJet device and performed assisted thrombolysis with urokinase without any complications, and simultaneously avoided unnecessary bowel resection.
In patients with symptomatic PVMVT without symptoms and signs of surgical abdomen, mechanical thrombectomy-assisted thrombolysis via an endovascular approach may be a safe and effective treatment option. This approach enables rapid debulking of the thrombus and facilitates rapid recanalization of mesenteric and portal venous flow, which results in prompt subsidence of symptoms and prevents unnecessary bowel resection. This approach can also reduce potential bleeding complications in compromised patient by decreasing the duration and amount of thrombolytic agent used [2,3,4,5]. However, more clinical data of the outcomes achieved using percutaneous mechanical thrombectomy-assisted thrombolysis to treat PVMVT are required; further long term follow-up and clinical studies are needed to determine the feasibility of an endovascular approach for treating this potentially fatal disease.
Figures and Tables
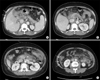 | Fig. 1Initial CT scan. Intraluminal thrombosis in the portal vein (A, arrow), splenic vein (B, arrows), and superior mesenteric vein (C, arrow) is evident. (D) Jejunal loop dilation and diffuse wall thickening (arrows) with a small amount of ascites are evident (dotted arrows; A, D). |
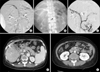 | Fig. 2(A) Portogram and mesenteric venogram showed extensive thrombosis in the portal vein and mesenteric vein. (B) AngioJet Spiroflex thrombectomy catheter in SMV (arrow). (C) The completion venogram demonstrated partial recanalization of venous flow in the portal vein and SMV and splenic vein. (D, E) Follow-up abdominal CT scan at the 16th hospital day. Improved blood flow with a small amount of residual intraluminal thrombus in the PV and SMV compared to the initial CT. (D) The puncture site of the liver was coil embolized (arrow). (E) Bowel loop dilatation and wall thickening also improved significantly. |
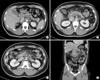 | Fig. 3Initial CT scan showed intraluminal thrombosis in the portal vein (A, arrow), splenic vein (B, arrows), and superior mesenteric vein (C, arrow). (C, D) Streaky infiltrations in the mesentery raised the suspicion of mesenteric edema (dotted arrows). |
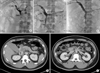 | Fig. 4(A) Venogram demonstrated extensive thrombosis in the portal vein (PV), superior mesenteric vein (SMV), and splenic vein (SV). (B) Using AngioJet catheter (arrow), mechanical thrombectomy was performed in the thrombosed segments of superior mesenteric vein. (C) Two days after the procedure, venography of superior mesenteric and portal veins demonstrated partial recanalization of these vessels. (D, E) Postprocedure 8th day abdominal CT scan. The volume of thrombi in the portal vein (D, arrow) and superior mesenteric vein (E, arrow) was decreased, and mesenteric edema had improved. |
Table 1
Summary of reported case of mechanical thrombectomy-assisted thrombolysis for symptomatic PVMVT

PVMVT, portal vein and mesenteric vein thrombosis; PV, portal vein; SMV, superior mesenteric vein; SV, splenic vein; HD, hospital day; tPA, tissue plasminogen activator; SMA, superior mesenteric artery; TAH/BSO, total abdominal histerectomy and bilateral salpingo-oophorectomy; PTA, percutaneous angioplasty; SMV, superior mesenteric vein.
"~" indicates lack of information in the original case report.
References
1. Chen C. Direct thrombolytic therapy in portal and mesenteric vein thrombosis. J Vasc Surg. 2012; 56:1124–1126.
2. Lopera JE, Correa G, Brazzini A, Ustunsoz B, Patel S, Janchai A, et al. Percutaneous transhepatic treatment of symptomatic mesenteric venous thrombosis. J Vasc Surg. 2002; 36:1058–1061.
3. Zhou W, Choi L, Lin PH, Dardik A, Eraso A, Lumsden AB. Percutaneous transhepatic thrombectomy and pharmacologic thrombolysis of mesenteric venous thrombosis. Vascular. 2007; 15:41–45.
4. Kim HS, Patra A, Khan J, Arepally A, Streiff MB. Transhepatic catheter-directed thrombectomy and thrombolysis of acute superior mesenteric venous thrombosis. J Vasc Interv Radiol. 2005; 16:1685–1691.
5. Uflacker R. Applications of percutaneous mechanical thrombectomy in transjugular intrahepatic portosystemic shunt and portal vein thrombosis. Tech Vasc Interv Radiol. 2003; 6:59–69.
6. Rosen MP, Sheiman R. Transhepatic mechanical thrombectomy followed by infusion of TPA into the superior mesenteric artery to treat acute mesenteric vein thrombosis. J Vasc Interv Radiol. 2000; 11:195–198.
7. Wissgott C, Kamusella P, Andresen R. Percutaneous mechanical thrombectomy: advantages and limitations. J Cardiovasc Surg (Torino). 2011; 52:477–484.
8. Morgan R, Belli AM. Percutaneous thrombectomy: a review. Eur Radiol. 2002; 12:205–217.
9. Sharafuddin MJ, Hicks ME. Current status of percutaneous mechanical thrombectomy. Part I. General principles. J Vasc Interv Radiol. 1997; 8:911–921.
10. Sharafuddin MJ, Hicks ME. Current status of percutaneous mechanical thrombectomy. Part II. Devices and mechanisms of action. J Vasc Interv Radiol. 1998; 9:15–31.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download