Abstract
Purpose
Prostaglandin E2 (PGE2) is a contributory carcinogen in gastric adenocarcinoma. 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) catabolizes PGE2 by oxidizing its 15(s)-hydroxy group. The aim of this study was to investigate the expression of 15-PGDH in gastric adenocarcinoma tissue and the relationship between 15-PGDH expression and clinicopathologic features of gastric adenocarcinoma.
Methods
Ninety-nine patients who underwent surgical resection for gastric adenocarcinoma between January 2007 and December 2007 were enrolled and evaluated retrospectively.
Results
In 62 patients (62.6%), 15-PGDH expression was lower in gastric adenocarcinoma tissue than in nonneoplastic tissue. Regarding the relationship between 15-PGDH expression and clinicopathological features, 15-PGDH expression was significantly lower in tissues with poor differentiation (P = 0.002), advanced T stage (P = 0.0319), a higher number of lymph node metastases (P = 0.045), lymphatic invasion (P = 0.031), and vascular invasion (P = 0.036).
Prostaglandin is a lipid-protein complex derived from arachidonic acid that has various physiologic functions. Particularly, prostaglandin E2 (PGE2) is secreted from tumor cells of epithelial origin and plays an important role in tumor growth, including the modulation of neovascularization, tumor invasion, and tumor metastasis [1,2].
Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme in the production of PGE2 [1,3] and a relationship between COX-2 overexpression and tumor occurrence has been reported [4]. COX-2 inhibitors prevent precancer lesion development and malignant tumor occurrence by suppressing tumor growth and neovascularization, as well as inducing apoptosis [5]. However, the long-term use of COX-2 inhibitors can cause several complications, including myocardial infarction and stroke. Hence, most studies of COX-2 inhibitor use have been delayed or interrupted [6,7].
Recently, studies seeking COX-2 inhibitor substitutes have been conducted. Among them, studies concerning the use of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) have gained much attention. PGE2 is known to be a contributory carcinogen in gastric adenocarcinoma. 15-PGDH catabolizes PGE2 by oxidizing its 15(s)-hydroxy group [8]. 15-PGDH expression is reduced in colon, breast, prostate, lung, and medullary thyroid cancers [9,10,11,12,13]. Presently, several studies concerning the relationship between gastric adenocarcinoma and 15-PGDH expression have been performed, but conflicting results were reported [14,15,16,17,18,19,20,21].
The aim of this study was to investigate 15-PGDH expression in gastric adenocarcinoma tissue. Additionally, we researched the possibility of 15-PGDH as a prognostic factor by investigating the relationship between 15-PGDH expression and clinicopathologic features, including stage, lymphatic invasion, and vascular invasion, which are known prognostic factors of gastric adenocarcinoma.
A total of 273 patients underwent surgical resection for gastric adenocarcinoma. Of these patients, 99 who underwent D2 lymph node dissection and had preserved histopathologic samples, including cancerous and nonneoplastic tissues, were enrolled between January 2007 and December. We conducted a retrospective analysis of the clinicopathological features of the patients in a prospectively collected gastric cancer database. We identified the TNM stage of each tumor based on the American Joint Committee on Cancer 7th edition [22].
Cancer cells were identified by analysis of hematoxylin and eosin stained slides from the enrolled patients. Two main tissue samples (each 2.0 mm in diameter) were obtained from a chosen tissue paraffin block from the enrolled patients. Recipient paraffin blocks were then made from the tissue. Anti-human 15-PGDH rabbit polyclonal antibodies (1:300 dilution; Cayman Chemical, Ann Arbor, MI, USA) were used for immunopathological staining. A 4-µm-thick tissue section obtained from the tissue microarray block was cultivated in Tris-ethylenediaminetetraacetic acid buffer (pH 8.0) at 99℃ for 30 minutes.
The endogenous peroxidase activity of the tissue was removed by treatment with hydrogen peroxide. The tissue sections were processed using a UV blocker reagent (Ventana Medical Systems, Tuscon, AZ, USA). The sections were next cultivated at 37℃ for 30 minutes, followed by cultivation with an horseradish peroxidase multimer reagent (Ventana Medical Systems) for 8 minutes. Finally, the tissue sections were counterstained with Mayer's hematoxylin.
Immunohistochemical staining was performed using gastric cancer lesions and nonneoplastic lesions. A skilled pathologist then analyzed the stained tissue sections and scored 15-PGDH expression using a 4-point system as follows: 0, no staining; 1, dark-stained cytoplasm, <10% of the total cancer cells; 2, dark-stained cytoplasm, 10%-90% of the total cancer cells; and 3, dark-stained cytoplasm, >90% of the total cancer cells (Fig. 1).
Grade 0 or 1 15-PGDH expression that was lower in the gastric adenocarcinoma tissue than in the nonneoplastic tissue was classified as a loss of 15-PGDH expression. Grade 2 15-PGDH expression that was similar in the gastric adenocarcinoma and nonneoplastic tissues was classified as moderate 15-PGDH expression. Grade 3 15-PGDH expression that was higher in the gastric adenocarcinoma tissue than in the non-neoplastic tissue was classified as an increase in 15-PGDH expression.
Statistical tests were performed using SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA). An analysis of variance or the Kruskal-Wallis method was used for continuous variable analysis. A chi-squared test or Fisher exact test was used for categorical variables. A P-value < 0.05 was deemed to indicate statistical significance.
The sex ratio of the 99 patients who were enrolled was 75% male and 25% female. The mean age was 61.3 years (range, 36-84 years). Regarding the types of surgery that were performed, 70 patients underwent radical subtotal gastrectomy (70.7%), while 29 patients underwent radical total gastrectomy (29.3%). Patients with negative microscopic resection margins comprised 97 cases (97.98%), while patients with positive microscopic resection margins included 2 cases (2.02%).
Concerning the differentiation grade, 1 patient (1.01%) had papillary adenocarcinoma, 13 patients (13.13%) had well-differentiated tubular adenocarcinoma, 33 patients (33.33%) had moderately differentiated tubular adenocarcinoma, 21 patients (21.21%) had poorly differentiated tubular adenocarcinoma, 1 patient (1.01%) had mucinous adenocarcinoma, and 30 patients (30.30%) had signet ring cell carcinoma.
Regarding the T stage, 44 patients (44.45%) had T1, 12 patients (12.12%) had T2, 29 patients (29.29%) had T3, and 14 patients (14.14%) had T4 disease. Concerning the N stage, 59 patients (59.60%) had N0, 13 patients (13.13%) had N1, 9 patients (9.09%) had N2, and 18 patients (18.18%) had N3 disease. Regarding the TNM stage, 53 patients (53.54%) had stage I, 17 patients (17.17%) had stage II, 27 patients (27.27%) had stage III, and 2 patients (2.02%) had stage IV disease.
Concerning invasion, 52 patients (52.52%) had lymphatic invasion, 54 (54.55%) had vascular invasion, and 57 (57.58%) had neural invasion (Table 1).
A total of 62 patients (62.63%) showed no or low-level 15-PGDH expression (no expression: 22 cases; low-level expression: 40 cases), 27 patients (27.27%) showed moderate 15-PGDH expression, and 10 (1 patient, 0.1%) showed high-level 15-PGDH expression.
No correlation was found between 15-PGDH expression and sex (P = 0.472) and between 15-PGDH expression and age (P = 0.3153). A low differentiation grade and low-level 15-PGDH expression were significantly correlated (P = 0.002). A high T stage and low-level 15-PGDH expression were also significantly correlated (P = 0.032); however, low-level 15-PGDH expression was not significantly correlated with N stage (P = 0.109) and TNM stage (P = 0.166). Lymph node metastasis tends to increase 15-PGDH expression, but no significant correlation was noted between lymph node metastasis and 15-PGDH expression (P = 0.060). A significant correlation was found between the number of lymph node metastases and 15-PGDH expression (P = 0.045). 15-PGDH expression was significantly lower in tissues with lymphatic invasion (P = 0.031) and vascular invasion (P = 0.036). Neural invasion was also correlated with low-level 15-PGDH expression, but no statistical significance was noted (P = 0.059) (Tables 2, 3).
The relationship between gastric adenocarcinoma and overexpression of COX-2, which is the rate-limiting enzyme in prostaglandin production, has been reported in the literature [23,24,25,26]. However, the relationship between gastric adenocarcinoma and the expression of 15-PGDH, which catabolizes prostaglandins to a reduced 15-keto metabolite by oxidation of the 15(s)-hydroxy group, remains controversial. According to an existing study, 15-PGDH is related to tumor suppression, and 15-PGDH expression is decreased in lung, colon, breast, and prostate cancers [7,9,10,11,12].
Tatsuwaki et al. [14] reported 15-PGDH expression in 37 of 71 gastric adenocarcinoma cases (50.7%), and the survival rate with 15-PGDH expression was higher than that without 15-PGDH expression (P = 0.05). Jang et al. [17] analyzed 28 normal and 28 gastric cancer tissue samples and reported that 15-PGDH was overexpressed in normal tissue compared with gastric adenocarcinoma tissue (P < 0.05). In the present study, 15-PGDH expression in 62 of 99 gastric adenocarcinoma patients (62.6%) was lower than that in normal tissue. Contrary to these reports, Yoo et al. [16] reported 15-PGDH expression in 54 of 60 gastric adenocarcinoma cases (90%).
Conflicting results have been reported concerning the relationship between 15-PGDH expression and clinicopathologic features. Yoo et al. [16] reported that 15-PGDH expression was not associated with clinicopathologic features such as age, sex, lymph node metastasis, TNM stage, and differentiation. Additionally, Thiel et al. [18] reported low-level expression of 15-PGDH in gastric adenocarcinoma and that 15-PGDH expression was not associated with clinicopathologic features. Contrary to these reports, Liu et al. [19] reported that 15-PGDH expression was associated with differentiation, TNM stage, and lymph node metastasis. Tatsuwaki et al. [14] and Lou et al. [20] reported that 15-PGDH was associated with differentiation and stage.
In the current study, 15-PGDH expression was significantly lower in tissues with poor differentiation and advanced T stage. Tissues with lymph node metastasis showed a trend toward lower 15-PGDH expression, and 15-PGDH expression was significantly lower in tissues with a higher number of lymph node metastases. However, no significant correlation was found between 15-PGDH expression and clinicopathologic features such as N stage and TNM stage. These results are unexpected because 59.9% of the cancerous tissues were from patients with early gastric cancer without lymph node metastasis.
Therefore, further study of tissues from advanced gastric cancer patients, particularly those with lymph node metastasis, is necessary.
In the present study, we investigated the correlation between 15-PGDH expression and clinicopathologic features such as lymphatic invasion, vascular invasion, and nerve invasion. 15-PGDH expression was significantly lower in tissues with lymphatic invasion and vascular invasion. No significant correlation was found between 15-PGDH expression and nerve invasion, but 15-PGDH expression tended to be lower with nerve invasion.
The expression of 15-PGDH was associated with clinicopathologic features such as differentiation grade, T stage, lymphatic invasion, and vascular invasion.
In gastric adenocarcinoma, T stage, lymphatic invasion, and vascular invasion are known to be prognostic factors [27,28,29,30]. If the survival rate of the patients in this study were investigated further, 15-PGDH may be considered a prognostic factor.
In conclusion, in 62.6% of the patients with gastric adenocarcinoma, 15-PGDH expression was significantly lower in gastric adenocarcinoma tissues than in nonneoplastic tissues.
Concerning the relationship between the expression of 15-PGDH and clinicopathological features, 15-PGDH expression was significantly correlated with T stage, lymphatic invasion, and vascular invasion, which are known prognostic factors of gastric adenocarcinoma. Expression of 15-PGDH is also considered to be a prognostic factor. However, the present study is limited in that 59.9% of the patients were early gastric cancer patients, and the survival rates of these patients were not investigated.Future studies concerning the relationship between 15-PGDH expression and gastric adenocarcinoma, the role of 15-PGDH as a gastric prognostic factor, and the relationship between 15-PGDH and the survival rate of gastric adenocarcinoma patients are necessary.
Figures and Tables
Fig. 1
Immunohistochemical stain for 15-hydroxyprostaglandin dehydronase (15-PGDH). Normal gastric glands express 15-PGDH (A, ×100). Gastric cancer cells show variable expression of 15-PGDH: loss of expression (B, ×100), preserved expression (C, ×100), and overexpression (D, ×100).
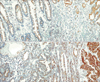
Table 2
The relationship of clinicopathologic characteristics with 15-PGDH expression (continuous variable)

References
1. Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001; 1:11–21.
2. Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006; 55:115–122.
3. Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004; 56:387–437.
4. Eisinger AL, Prescott SM, Jones DA, Stafforini DM. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat. 2007; 82:147–154.
5. Nam KT, Hahm KB, Oh SY, Yeo M, Han SU, Ahn B, et al. The selective cyclooxygenase-2 inhibitor nimesulide prevents Helicobacter pylori-associated gastric cancer development in a mouse model. Clin Cancer Res. 2004; 10:8105–8113.
6. Salzberg DJ, Weir MR. COX-2 inhibitors and cardiovascular risk. Subcell Biochem. 2007; 42:159–174.
7. Farooq M, Haq I, Qureshi AS. Cardiovascular risks of COX inhibition: current perspectives. Expert Opin Pharmacother. 2008; 9:1311–1319.
8. Tai HH, Cho H, Tong M, Ding Y. NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and biological functions. Curr Pharm Des. 2006; 12:955–962.
9. Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006; 66:7818–7823.
10. Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005; 26:65–72.
11. Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005; 280:3217–3223.
12. Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004; 101:17468–17473.
13. Tong M, Tai HH. Synergistic induction of the nicotinamide adenine dinucleotide-linked 15-hydroxyprostaglandin dehydrogenase by an androgen and interleukin-6 or forskolin in human prostate cancer cells. Endocrinology. 2004; 145:2141–2147.
14. Tatsuwaki H, Tanigawa T, Watanabe T, Machida H, Okazaki H, Yamagami H, et al. Reduction of 15-hydroxyprostaglandin dehydrogenase expression is an independent predictor of poor survival associated with enhanced cell proliferation in gastric adenocarcinoma. Cancer Sci. 2010; 101:550–558.
15. Liu Z, Wang X, Lu Y, Han S, Zhang F, Zhai H, et al. Expression of 15-PGDH is downregulated by COX-2 in gastric cancer. Carcinogenesis. 2008; 29:1219–1227.
16. Yoo NJ, Jeong EG, Lee SH, Lee SH. Expression of 15-hydroxyprostaglandin dehydrogenase, a COX-2 antagonist and tumour suppressor, is not altered in gastric carcinomas. Pathology. 2007; 39:174–175.
17. Jang TJ, Ji YS, Jung KH. Decreased expression of 15-hydroxyprostaglandin dehydrogenase in gastric carcinomas. Yonsei Med J. 2008; 49:917–922.
18. Thiel A, Ganesan A, Mrena J, Junnila S, Nykanen A, Hemmes A, et al. 15-hydroxyprostaglandin dehydrogenase is down-regulated in gastric cancer. Clin Cancer Res. 2009; 15:4572–4580.
19. Liu Z, Wang X, Lu Y, Du R, Luo G, Wang J, et al. 15-Hydroxyprostaglandin dehydrogenase is a tumor suppressor of human gastric cancer. Cancer Biol Ther. 2010; 10:780–787.
20. Lou LH, Jing DD, Lai YX, Lu YY, Li JK, Wu K. 15-PGDH is reduced and induces apoptosis and cell cycle arrest in gastric carcinoma. World J Gastroenterol. 2012; 18:1028–1037.
21. Song HJ, Myung SJ, Kim IW, Jeong JY, Park YS, Lee SM, et al. 15-hydroxyprostaglandin dehydrogenase is downregulated and exhibits tumor suppressor activity in gastric cancer. Cancer Invest. 2011; 29:257–265.
22. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer;2010.
23. Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997; 57:1276–1280.
24. Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, et al. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999; 94:451–455.
25. Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000; 6:519–525.
26. Jang TJ. Expression of proteins related to prostaglandin E2 biosynthesis is increased in human gastric cancer and during gastric carcinogenesis. Virchows Arch. 2004; 445:564–571.
27. Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005; 241:27–39.
28. Tanaka A, Watanabe T, Okuno K, Yasutomi M. Perineural invasion as a predictor of recurrence of gastric cancer. Cancer. 1994; 73:550–555.
29. Kooby DA, Suriawinata A, Klimstra DS, Brennan MF, Karpeh MS. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003; 237:828–835.
30. Kim CH, Jang SW, Kang SH, Kim SW, Song SK. The significance of lymphatic, venous, and neural invasion as prognostic factors in patients with gastric cancer. J Korean Gastric Cancer Assoc. 2005; 5:113–119.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download