Abstract
Purpose
The aim of this study was to evaluate the surgical outcomes of laparoscopic approach for hiatal hernia (HH) in pediatric patients.
Methods
This was a retrospective study of 33 patients younger than 18 years who underwent an operation for HH between January 1999 and December 2012.
Results
The HH symptoms were various and included regurgitation, vomiting, weight loss, cough, hoarseness, and cyanosis. Among the 33 patients, there were 25 sliding types, 1 paraesophageal type, and 7 mixed types. Open surgery (OS) and laparoscopic surgery (LS) were used in 16 and 17 patients, respectively. There were no statistically significant differences in sex, age, or body weight between the groups. The median operating time was longer in the LS group (150 minutes; range, 90-250 minutes vs. 125 minutes; range, 66-194 minutes; P = 0.028). Time to oral intake was shorter in the LS group than in the OS group (1 day; range, 1-3 days vs. 2 days; range, 1-7 days; P = 0.001) and time to full feeding was shorter in the LS group than in the OS group (6 days; range, 3-16 days vs. 10 days; range, 3-33 days; P = 0.048). There were no differences in length of hospital stay and complications between the two groups. There was no perioperative mortality or recurrence of HH.
A hiatal hernia (HH) is a stomach herniation located in the posterior mediastinum through the esophageal hiatus that is rarely found in pediatric patients. Bowditch first used the term HH in 1853, and Soresi performed the first operation as a reduction of the stomach and approximation of the crura in 1919 (quoted from [1,2]). HHs are mostly congenital in origin and are associated with various abnormalities in diaphragm development in pediatric patients and the cause of HH in adults is gradual enlargement of the hiatus, congenital defect and post-traumatic damage [1,3]. There are four types of HHs: sliding HHs (type 1), paraesophageal hernias (PEHs; type 2), mixed hernias (type 3), and complex PEHs (type 4) (Fig. 1) [4]. Mixed hernias involve combinations of sliding HHs and PEHs, and sliding and PEHs combined with other organic HHs are classified as type 4. Types 2, 3, and 4 are clinically classified as PEHs [4,5,6].
Laparoscopic HH repair with fundoplication is performed as a choice of treatment for HHs in our institute. Although, laparoscopic hernia repair with fundoplication for HHs is established as the treatment of choice in adults, well-designed studies in children are limited by its rarity. The aim of this study was to compare the surgical outcomes of laparoscopic hernia repair with that of laparotomy in children.
Patients with HH's who underwent open or laparoscopic hernia repair with fundoplication in the Division of Pediatric Surgery, Asan Medical Center between January 1999 and December 2012 were included. This study contains data from three surgeons. Only patients under the age of 18 years old were included. All medical records were retrospectively reviewed and the following patient data were collected: age, sex, presenting symptoms, radiological studies, hospital stay, operation time, morbidity, and mortality.
In our institution, medical treatment was performed for patients with small sliding hernias. No uniform definition exists for large HHs, although some authors define them as herniation of more than 30% of the stomach [2]. Surgical treatment was employed for patients with sliding hernias who had no symptomatic improvement after medical treatment, those with large sliding hernias, or those who needed gastrostomy and/or fundoplication for tube feeding due to combined disease. For HHs of types 2, 3, and 4, surgical treatment should be performed given the possibility and severity of complications.
Our basic operative technique for HHs is composed of the reduction of herniated organs, crural repair, and fundoplication. Hernial sacs were removed for type 2 and type 3 HHs, not for small type 1 HHs. When hernial sacs comprised less than one third of the stomach, complete excision of the hernial sac was not performed; instead, the hiatal rim was trimmed. The hiatus was repaired by using interrupted sutures of nonabsorbable suture material (4-0 Prolene; Ethicon, Cincinnati, OH, USA). Before the first laparoscopic surgery (LS) for a HH was performed at our institution in 2001, there had been seven cases of open surgery (OS) for HHs. Of three surgeons, one performed LSs, and all three surgeons had OS cases. Thal and Nissen fundoplications were performed during OS according to surgeon's preference and Nissen fundoplication was routinely performed in LS due to its ease of use in laparoscopic conditions. Recently, Thal fundoplication and Nissen fundoplication have been used for additional treatment of HH's in OS.
In the laparoscopic procedure, a pneumoperitoneum at 10-12 mmHg by CO2 gas is established in the umbilical region with a 5-mm trocar placed in the lower umbilical region. After confirming the intraperitoneal cavity by 30° laparoscopy, the main working trocar is inserted in the right side of the patient and another 5-mm trocar for the operator is inserted in the left side of the patient. The first assistant uses the 5-mm trocar inserted in the right subcostal area of the patient for traction of the liver (Fig. 2). For HHs, 4 trocars (5 mm) are routinely used. However, when traction of the stomach is needed, an additional 2- or 3-mm trocar is used. Complete reduction of the herniated organ is first performed and the hernial sac is removed. Tension-free crural repair is performed with 3-0 or 4-0 Ethibond (Ethicon). Fundoplication is the next step. If necessary, short gastric vessels are dissected and ligated. For fundoplications, 4-0 Prolene or 3-0 Ethibond are used.
Dietary intake was scheduled to recommence upon gas passage. Simple abdomen x-ray images, amount of gastric drain, and bowel sounds were checked daily. Only sipping of water was permitted on the first day of the diet. On the second day of the diet, a liquid diet or 50%-diluted milk was served. If tolerable, patients were permitted a soft diet or undiluted milk on the third day. The diet amount was increased according to the diet tolerability of the patients. Data collected from the out-patient office was used in the analysis of outcomes and included a barium esophagography protocol in most patients. Anatomic failure was defined as a recurrent HH, intrathoracic wrap migration, or unwrapping of the fundoplication on follow-up barium esophagography.
Summary data were formatted as the median (range) or number of patients (percentage of population). PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The Mann-Whitney U test and the Wilcoxon test were used to assess the significance of the nonparametric data sets. The significance level was set at a P-value of less than 0.05.
A total of 33 children (17 males) were included in these analyses. The median age was 8.0 months (range, 0.2-192 months) and the median body weight was 6.5 kg (range, 2.1-28.5 kg). Three patients were neonates and 16 patients were infants. Of the three HH types found, the most common was type 1 (sliding), which was found in 25 patients (75.8%). There was no type 4 HHs in our study cohort. In our current study, HH was initially detected by chest x-ray (n = 6, 18.2%), computed tomography (n = 4, 12.1%), esophagogastroscopy (n = 1, 3.0%), or esophagography (n = 22, 66.7%). The diagnosis of HH was confirmed by esophagography.
The most common symptom of HH was regurgitation and/or intermittent vomiting (n = 21, 63.6%) and atypical symptoms of gastroesophageal reflux disease (GERD), including cough, hoarseness, and cyanosis (with dyspnea), were present in nine patients (27.3%). Ten patients (30.3%) had a history of pneumonia. Interestingly, two patients failed to thrive despite without any gastrointestinal symptoms. Six patients (18.2%) had no gastrointestinal or respiratory symptoms. Fundoplication and gastrostomy was needed in eight patients who had neurological deficits (n = 6) or severe cardiac anomalies (n = 2).
We classified the patients into two groups: the OS group and the LS group (Table 1). Moreover, we used two types of fundoplications-Nissen and Thal fundoplications-and also performed pyloroplasty depending on the possibility of perioperative injury to the vagus nerve. There were no statistically significant differences in the demographic findings (sex, age, body weight, and birth weight) and the type of HH between the OS and LS groups (Table 2).
Resumption of oral intake (P = 0.001) and a full amount of feeding (P = 0.048) began sooner in patients of the LS group than the OS group. The median hospital stay was 18 days (range, 5-150 days) for the OS group and 11 days (range, 4-50 days) for the LS group (P = 0.564) (Table 3). The number of complications were two and one for the OS and LS groups, respectively (P = 0.465). All complications were wound infections. In our perioperative findings, the median duration of the operation was found to be longer in the LS group than in the OS group (P = 0.028). During the follow-up period, only one patient who had undergone an open Nissen fundoplication without pyloroplasty showed symptoms of dysphagia (Table 3). However, symptoms were relieved one month after the operation.
Sixteen of our patients (48.5%) had concomitant diseases. Neurological problems played an important role in prolonging hospital stays and delaying time to full feeding. Six patients had concomitant neurological problems, including epilepsy (n = 2), cerebral palsy (n = 1), quadriplegia (n = 1), Menkes disease (copper transport disease, n = 1), and hydranencephaly (n = 1) (Table 2). One case of HH developed after the patient underwent an esophagoesophagostomy for long-gap esophageal atresia. Two patients had concomitant Bochdalek's hernias at the right side of the diaphragm. One patient who was found to have a small lesion during surgery underwent synchronous repair of Bochdalek's hernia, and one patient diagnosed with HH had undergone laparoscopic repair of Bochdalek's hernia 6 months earlier.
Seven patients had congenital cardiac anomalies, including a ventricular septal defect (n = 1), patent ductus arteriosus (n = 2), total anomalous pulmonary venous return (n = 1), and tetralogy of Fallot (n = 3). We experienced three deaths among our cohort, which were not perioperative mortalities associated with HH surgeries but were due to complications arising from surgeries for cardiac anomalies. The causes of death were sepsis, pneumonia, and recurrent bowel perforation after cardiac surgery, respectively. With regard to the recurrent bowel perforation case, these developed 10 days after cardiac surgery for which a small bowel resection and ileostomy was performed. However, two weeks later, a recurrent bowel perforation developed and the patient succumbed to sepsis. We had no HH recurrences among our patients but there was one case of reoperation. In this case, we performed a hernial repair and Thal fundoplication using an open procedure. However, 3 years after this further operation, GERD developed due to unwrapping of the fundoplication (but not HH). We then performed a Nissen fundoplication with a laparotomy for this patient.
Sliding hernia (type 1), observed in approximately 95% of HHs, is caused by weakness of the phrenoesophageal membrane around the esophagus. The stomach is partially herniated, and the gastroesophageal (GE) junction moves up and becomes located at the mediastinum [6,7]. PEH (type 2) is characterized as a part of the stomach that is herniated into the intrathoracic area while the GE junction and stomach cardia is located under the diaphragm. The incidence of PEH is reported to be between 3.5% and 5% among all operated HHs. Mixed hernias (type 3) are a combination of type 1 and type 2 HHs, and type 4 HHs are a multiple HH that involves herniation of peritoneal organs, such as the colon, intestine, spleen, and pancreas, and type 2 or type 3 HHs [7,8].
The widely accepted management of HH consists of surgical repair that involves reduction of the herniated organ, excision of the hernial sac, approximation of the crura, and an antireflux procedure where indicated. Although many kinds of fundoplication have been reported as treatments for HHs in adults, there have been few reports of HHs in pediatric patients [1,8,9]. Yagi et al. [10] previously described four patients aged between 30 days and 14 months, and van der Zee et al. [11] presented two patients of 9 and 14 months of age with PEH, all of whom underwent successful laparoscopic hernia reduction, hiatal repair, and fundoplication.
We performed fundoplications in all of the cases included in our present analyses because we believe that this method can reduce the recurrence of HH. Although symptoms of HH may resolve after crural repair alone, fundoplication helps secure the stomach intraabdominally, by increasing the intraabdominal esophageal length, and may be necessary to prevent postoperative reflux secondary to preexisting anatomic abnormalities [12]. We performed two kinds of fundoplication-Thal and Nissen-as antireflux surgical procedures. Nissen fundoplication is the most widely performed but is associated with a high incidence of postoperative dysphagia and an appreciable recurrence rate in children with disabilities [13,14,15,16]. Thal is a partial wrap and is also widely performed, and is reported to be a simple and safe operative treatment in children with fewer perioperative complications and better long-term outcomes [17,18]. Surgical outcomes of both Thal and Nissen fundoplications as additional antireflux procedures for HH were good among our current study patients and there was no recurrence of HH in this series. We thus believe that hiatal repair with fundoplication is the best surgical option for preventing recurrence of HH in children.
Esposito et al. [13] have previously reported the following rates of dysphagia: 4 in 94 patients (4.3%) who received laparoscopic Nissen fundoplications and 1 in 48 patients (2.1%) who received Thal fundoplications. Kubiak et al. [17] reported dysphagia rates of 13.5% and 11.6% for each respective fundoplication, but the rates of severe dysphagia for Nissen and Thal were 10.1% and 1.7%, respectively (P = 0.018) in that study. In our current study, we experienced only one dysphagia cases among the HH patients. However, our present analysis did not encompass all of the data of fundoplication from our center and we have to review all data including GERD patients at our institution in the future to clarify the outcomes of fundoplications.
All of the types 2 and 3 patients had large-sized hernial sacs that were large enough to enable herniation of almost all of the stomach through the hernial defect. Including 5 patients with types 1 and 8 patients with types 2 and 3 hernias, 13 patients (39.4%) had large-sized defects. Thus, among 33 cases, we had 13 cases of hernial sac resection. Among these, laparoscopic repair was performed in eight patients (three cases of type 1, one case of type 2, and four cases of type 3). We had no need to apply an artificial patch or mesh when performing surgical repair of large-sized HHs. Many previous reports described the safety and the feasibility of laparoscopic HH repair with biological or synthetic mesh in elder patients [19,20,21,22]. We tried to repair the large HH in children without mesh, because the defect in children is relatively smaller than in adult patients.
Fullum et al. [3] reported in their nationwide study that laparoscopic approach to HH was associated with a lower mortality in the uncomplicated group. However, they described that numerous studies have demonstrated the safety, efficacy, and durability of LS for HH, but its acceptance as the procedure of choice had not been universal, and no randomized controlled trials comparing the open versus laparoscopic approach exist. In pediatric population, the proof of feasibility and safety of laparoscopic approach for HH was not established too. This current study will add some helpful information to perform laparoscopic approach to HH.
The surgical outcomes of LS and OS are good in our experience, and LS was found to have some advantages over OS, namely a shorter time to oral intake and a shorter time to full feeding. We experienced no recurrence of HH following laparoscopic hernia repair with fundoplication in our pediatric series. In conclusion, as a treatment for HH in pediatric patients, laparoscopic hernia repair with fundoplication is safe and feasible and could be an optimal and practical choice of treatment. Further randomized studies will be required in the future to establish whether LS for HH could become the standard treatment option.
Figures and Tables
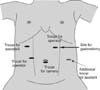
Fig. 2
Location of trocars for laparoscopic approach to hiatal hernia in children. We usually use 5-mm trocars and laparoscopic devices for laparoscopic surgery for hiatal hernia. However, we use 3-mm trocars for neonates and infants. The trocar pointed with the normal arrow is used as the main working port for the operator, and is also used as the gastrostomy site at the end of surgery. The trocar pointed with dashed arrow is used for traction of stomach, if necessary.
References
1. Stylopoulos N, Rattner DW. The history of hiatal hernia surgery: from Bowditch to laparoscopy. Ann Surg. 2005; 241:185–193.
2. Mitiek MO, Andrade RS. Giant hiatal hernia. Ann Thorac Surg. 2010; 89:S2168–S2173.
3. Fullum TM, Oyetunji TA, Ortega G, Tran DD, Woods IM, Obayomi-Davies O, et al. Open versus laparoscopic hiatal hernia repair. JSLS. 2013; 17:23–29.
4. Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg. 1967; 53:33–54.
5. Lee JW, Kim HS, Ryu BY, Kim HK, Han SJ. Paraesophageal hernia with small bowel strangulation. J Korean Surg Soc. 2010; 78:195–198.
6. Jang WN, Park IS, Park KW, Yoo SY, Lee J, Cho SH. A case of congenital paraesophageal hiatal hernia in infancy. Pediatr Gastroenterol Hepatol Nutr. 2012; 15:100–104.
7. Hashemi M, Sillin LF, Peters JH. Current concepts in the management of paraesophageal hiatal hernia. J Clin Gastroenterol. 1999; 29:8–13.
8. Weber C, Davis CS, Shankaran V, Fisichella PM. Hiatal hernias: a review of the pathophysiologic theories and implication for research. Surg Endosc. 2011; 25:3149–3153.
9. Aly A, Munt J, Jamieson GG, Ludemann R, Devitt PG, Watson DI. Laparoscopic repair of large hiatal hernias. Br J Surg. 2005; 92:648–653.
10. Yagi M, Nose K, Yamauchi K, Nogami T, Yoshida H, Okuyama H, et al. Laparoscopic intervention for intrathoracic stomach in infants. Surg Endosc. 2003; 17:1636–1639.
11. van der Zee DC, Bax NM, Kramer WL, Mokhaberi B, Ure BM. Laparoscopic management of a paraesophageal hernia with intrathoracic stomach in infants. Eur J Pediatr Surg. 2001; 11:52–54.
12. Bradley T, Stephenson J, Drugas G, Avansino JR. Laparoscopic management of neonatal paraesophageal hernia with intrathoracic gastric volvulus. J Pediatr Surg. 2010; 45:E21–E23.
13. Esposito C, Montupet P, van Der Zee D, Settimi A, Paye-Jaouen A, Centonze A, et al. Long-term outcome of laparoscopic Nissen, Toupet, and Thal antireflux procedures for neurologically normal children with gastroesophageal reflux disease. Surg Endosc. 2006; 20:855–858.
14. Esposito C, Montupet P, Reinberg O. Laparoscopic surgery for gastroesophageal reflux disease during the first year of life. J Pediatr Surg. 2001; 36:715–717.
15. Fernando HC, Luketich JD, Christie NA, Ikramuddin S, Schauer PR. Outcomes of laparoscopic Toupet compared to laparoscopic Nissen fundoplication. Surg Endosc. 2002; 16:905–908.
16. Kubiak R, Andrews J, Grant HW. Long-term outcome of laparoscopic nissen fundoplication compared with laparoscopic thal fundoplication in children: a prospective, randomized study. Ann Surg. 2011; 253:44–49.
17. Kubiak R, Andrews J, Grant HW. Laparoscopic Nissen fundoplication versus Thal fundoplication in children: comparison of short-term outcomes. J Laparoendosc Adv Surg Tech A. 2010; 20:665–669.
18. Ashcraft KW, Goodwin CD, Amoury RW, McGill CW, Holder TM. Thal fundoplication: a simple and safe operative treatment for gastroesophageal reflux. J Pediatr Surg. 1978; 13(6D):643–647.
19. Li J, Rosenthal RJ, Roy M, Szomstein S, Sesto M. Experience of laparoscopic paraesophageal hernia repair at a single institution. Am J Surg. 2012; 204:60–65.
20. Nason KS, Luketich JD, Witteman BP, Levy RM. The laparoscopic approach to paraesophageal hernia repair. J Gastrointest Surg. 2012; 16:417–426.
21. Wassenaar EB, Mier F, Sinan H, Petersen RP, Martin AV, Pellegrini CA, et al. The safety of biologic mesh for laparoscopic repair of large, complicated hiatal hernia. Surg Endosc. 2012; 26:1390–1396.
22. Furnée E, Hazebroek E. Mesh in laparoscopic large hiatal hernia repair: a systematic review of the literature. Surg Endosc. 2013; 27:3998–4008.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download