Abstract
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm of the gastrointestinal tract. Several recent findings that there are activating mutations in the KIT and PDGFRA (platelet-derived growth factor receptor-α) genes of GISTs provide the rationale for using targeted therapies such as imatinib or sunitinib. Sunitinib, an oral multitargeted receptor tyrosine kinase inhibitor that inhibits kinases such as KIT, PDGFR (platelet-derived growth factor recepter), and VEGFR (vascular endothelial growth factor receptor), was recently approved for the treatment of imatinib-refractory GIST. Sunitinib is generally well tolerated and has an acceptable toxicity profile; an adverse event such as bowel perforation is rare. We present a patient with imatinib-refractory GIST who was successfully treated using sunitinib, but developed bowel perforation. The mechanism involved in bowel perforation associated with sunitinib is unknown. However, we presume that in our patient, the dramatic reduction in disseminated peritoneal metastases and bowel invasion of recurrent GIST during sunitinib treatment might have resulted in the bowel perforation.
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm of the gastrointestinal tract. Most of these tumors (60%-70%) present in the stomach. The treatment of choice is surgical excision of the tumor and resection of infiltrated tissue. Prior to the year 2000, there was no known effective therapy for unresectable or metastatic GIST, because these tumors are extremely resistant to conventional cytotoxic chemotherapy and radiotherapy [1]. The discovery that these tumors express the CD117 antigen has led to substantial advances in the understanding of their biology. We know that GISTs usually have activating mutations in either KIT (75%-80%) or the platelet-derived growth factor receptor-α gene (PDGFRA) (5%-10%), which encode 2 closely related receptor tyrosine kinases; therefore, these mutations provide a rationale for the use of targeted therapies [2,3,4]. Because imatinib mesylate (Gleevec, Novartis Pharma, Basel, Switzerland), an inhibitor of KIT and PDGFRA tyrosine kinases, is now available for the treatment of metastatic and unresectable GISTs, it is important to understand the natural history of these tumors [1,2]. Although imatinib has greatly improved the survival of patients with advanced GIST, primary refractoriness or secondary resistance to imatinib therapy occurs in the majority of patients [1,2].
Currently, the only approved second-line drug is sunitinib malate, which was approved in 2006 by the U.S. Food and Drug Administration for the treatment of GIST patients following progression or resistance to imatinib [4]. Sunitinib malate (Sutent, Pfizer, New York, NY, USA) is an oral multitargeted tyrosine kinase inhibitor of KIT, platelet-derived growth factor recepter (PDGFR), all 3 isoforms of vascular endothelial growth factor receptor (VEGFR-1, VEGFR-2, VEGFR-3), Fms-like tyrosine kinase-3 receptor, and the receptor encoded by the ret protooncogene (RET). The mechanism of sunitinib activity against GIST is similar to imatinib [3,4]. Moreover, sunitinib exerts an additional antiangiogenic activity because it inhibits the activation of VEGFR. Because of this activity, sunitinib has been thought to be beneficial for patients with imatinib-resistant GIST [3,5].
The most common treatment-related adverse events of sunitinib have been reported to be fatigue, diarrhea, skin discoloration, nausea, mucositis, arterial hypertension, hand-and-foot syndrome, decreased left ventricular ejection fraction, and hypothyroidism. However, sunitinib is generally well tolerated and has an acceptable toxicity profile [3,4]. The overall rate of gastrointestinal perforation associated with sunitinib is unknown, since only a few cases have been reported from trials and case reports, and the mechanism of bowel perforation from sunitinib is unknown [5,6].
Here, we present an extremely rare case of bowel perforation associated with sunitinib therapy for GIST.
A 78-year-old woman with an abdominal mass for more than 1 month was admitted to Chosun University Hospital. Physical examination revealed a large mass in the periumbilical area. Abdominal computed tomography revealed an 18 cm abdominal mass with extensive central necrosis. She underwent complete resection of the mass, with total gastrectomy, distal pancreatectomy, splenectomy, and transverse colectomy (Fig. 1). Postoperative histopathologic examination showed a gastric GIST with negative resection margins and no metastatic lymph nodes. The mass was a 15 cm × 9 cm gastric GIST with 14 mitoses/50 high-power fields, and was categorized as a high-risk tumor. Immunostaining showed that the tumor was positive for C-KIT and CD34, and negative for S100 expression (Fig. 2). Adjuvant chemotherapy using oral imatinib mesilate was recommended to the patient. However, she declined treatment because it was too expensive.
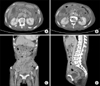
Eight months after the operation, the patient was found to have recurrent GIST involving the hilum and subcapsular region of the right lobe of the liver and peritoneal nodules. Imatinib (400 mg/day) was started as palliative treatment (Fig. 3A, B). At a 3-month follow-up, the liver masses had decreased and imatinib was found to be well tolerated by the patient, who continued on the same dose of iminitib (Fig. 3C, D). Follow-up imaging 6 months later showed peritoneal seeding in the region of the distal pancreas and a small amount of left pleural effusion and ascites (Fig. 3E, F). The patient refused second-line treatment, and imatinib therapy was continued despite the progression. Two months later, she was admitted because of severe dyspnea due to a large amount of pleural effusion and ascites (Fig. 4A). She requested second-line palliative oral medication without undergoing additional diagnostic evaluation.
Before she received sunitinib, a complete blood count (CBC) showed the following: white blood cell (WBC) count, 2,990 × 103/µL; neutrophils, 65% (1,943 × 103/µL); hemoglobin, 9.1 g/dL; and platelets, 222 × 103/µL. Partial thromboplastin time (PTT) and activated partial thromboplastin time (aPTT) were in the normal range. The patient's grade I anemia and neutropenia were due to imatinib toxicity. Examination of the pleural effusion revealed the following: WBC, 1,296/m3 (polymorphonuclear cells, 60%); pH, 7.20; lactate dehydrogenase (LDH), 1,266 U/L; and atypical cells. Cultures of the pleural effusate were negative. The results of these laboratory tests suggested malignant pleural effusion.
The patient's therapy was changed to oral sunitinib administered at 50 mg/day in cycles consisting of 4 weeks of sunitinib followed by 2 weeks of rest. On day 7 of the first cycle of sunitinib therapy, the volume of the patient's pleural effusion was unchanged, but her dyspnea had improved, and she requested discharge from the hospital. On day 21, sunitinib was found to be well tolerated except for grade I neutropenia (WBC, 2,390 × 103/µL; neutrophils, 1,769 × 103/µL) with markedly decreased pleural effusion and ascites (Fig. 4B). Oral sunitinib was continued at 50 mg/day.
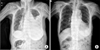
On day 26 of the first cycle of sunitinib therapy, the patient had a sudden onset of sharp, generalized abdominal pain. An imaging study revealed intraperitoneal free gas with many bubbles near the distal pancreatic region, suggesting small bowel perforation (Fig. 5), and the patient was hospitalized. The admission CBC showed the following: WBC, 1.19 × 103/µL; neutrophils, 63%; hemoglobin, 10.6 g/dL; and platelet count, 89 × 103/µL. The PT and aPTT were 10.9 and 25.6 seconds, respectively. She was diagnosed with generalized peritonitis due to bowel perforation and grade III neutropenia (750 × 103/µL) due to sunitinib toxicity. We recommended emergency surgery; however, the patient's guardian refused consent. The patient expired the next day.
Bowel perforation due to the toxicity of chemotherapeutic agents is a serious adverse event, and perforation is known to occur in patients with a chemosensitive malignancy such as gastrointestinal lymphoma [7]. Knowledge of the numerous intracellular signaling pathways involved in tumorigenesis enabled the identification of several potential molecular targets for the therapy of solid tumors. However, adverse events of new targeted therapies, such as bowel perforation and tumor rupture, are new challenges for the surgical oncologist [5]. The overall rate of gastrointestinal perforation associated with sunitinib is unknown [5,6]. One study found that emergency surgery for hemorrhage, tumor perforation, or abscess was needed in 3%-9% of patients receiving second-line therapy with sunitinib [5]. However, gastrointestinal (GI) perforation has been reported in <1% of patients taking sunitinib, and there was an extremely rare case of bowel perforation during sunitinib treatment for GIST [4,8].
The mechanism of bowel perforation associated with sunitinib toxicity is also unknown. Sunitinib has an additional antiangiogenic activity because it inhibits the activation of VEGFR such as bevacizumab. Bevacizumab, the first angiogenesis inhibitor approved for use in the United States, is a humanized anti-VEGF monoclonal antibody well known to be associated with the adverse event of GI perforation. The mechanism causing bowel perforation has also not been established; however, several mechanisms of action have been postulated [9].
Hypothetical mechanisms for GI perforation in patients treated with chemotherapy (including new targeted therapies) include the following: First, these drugs, such as bevacizumab, sunitinib, and sorafenib, block the VEGF receptors on endothelial cells and have a significant impact on the capillary beds of small intestinal villi. Such inhibition was found to significantly reduce the capillary density within these intestinal villi. Hence, antiangiogenic drugs may contribute to the development of microperforations within the intestinal wall secondary to the reduced regenerative capacity of the intestinal mucosa because of decreased blood vessel density. Second, in a variety of localized pathologic conditions, such as tumor-associated peptic ulcer disease, diverticulitis, or colitis, the intraluminal pressure is increased by tumor obstruction. These proposed risk factors may predispose bowel perforation in patients treated with chemotherapy (especially, antiangiogenic therapy). Third, bowel ischemia may be due to various thromboembolic events after antiangiogenic therapy. And last, rapid tumor necrosis as a result of chemotherapy or new targeted therapies may lead to bowel perforation in patients with bowel metastasis or invasion [5,6,7,8,9,10].
A mechanism for the perforation occurring in our patient during administration of sunitinib as a second-line therapy might have been the rapid death of tumor invading the GI mucosa. The cytotoxic and antiangiogenic activity of sunitinib may have resulted in a dramatic tumor response [3,4,6,8,10].
In conclusion, we presented an extremely rare case of bowel perforation associated with sunitinib therapy for imatinib-refractory GIST. Although we could not obtain pathologic confirmation through emergency surgery, we postulate that the dramatic reduction during sunitinib treatment for disseminated peritoneal metastases and bowel invasion in our patient with recurrent GIST of gastric origin, led to bowel perforation.
Figures and Tables
Fig. 1
(A, B) Abdominal computer tomography revealed an 18-cm size huge mass with extensive central necrosis in the abdomen. (C, D) Resected specimens showed large gastrointestinal stromal tumor mass with adhesion of stomach, small bowel, transverse colon, distal pancreas and spleen.
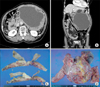
Fig. 2
The pathologic finding. (A) Spindle-shaped (arrow) tumor cells are composing bundles or fascicles (H&E, ×200). (B) Multiple mitosis (arrows) are also noted (H&E, ×400). (C) Immunohistochemically, the tumor was positive for CD34 (×100). (D) C-kit (×100).
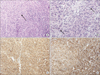
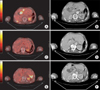
Fig. 3
(A, B) The gastrointestinal stromal tumor relapsed on the liver hilum and subcapsular area of the right lobe of the liver with peritoneal nodules. (C, D) Three months later of imatinib treatment, the mass of the liver hilum and liver capsule was decreased. (E, F) Follow-up image showed the recurrence of a peritoneal seeding mass in distal pancreatic area and a small amount of left pleural effusion and ascites.
References
1. Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007; 369:1731–1741.
2. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–480.
3. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006; 368:1329–1338.
4. Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007; 12:107–113.
5. Rutkowski P, Ruka W. Emergency surgery in the era of molecular treatment of solid tumours. Lancet Oncol. 2009; 10:157–163.
6. Coriat R, Ropert S, Mir O, Billemont B, Chaussade S, Massault PP, et al. Pneumatosis intestinalis associated with treatment of cancer patients with the vascular growth factor receptor tyrosine kinase inhibitors sorafenib and sunitinib. Invest New Drugs. 2011; 29:1090–1093.
7. Kim HG, Jung MR, Kang H, Cheong O, Ju JK, Park YK, et al. Discrepant bowel perforation from a primary lesion after chemotherapy of diffuse large B cell lymphoma. J Korean Surg Soc. 2009; 76:61–65.
8. Hur H, Park AR, Jee SB, Jung SE, Kim W, Jeon HM. Perforation of the colon by invading recurrent gastrointestinal stromal tumors during sunitinib treatment. World J Gastroenterol. 2008; 14:6096–6099.
9. Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007; 96:1788–1795.
10. Ruka W, Rutkowski P, Szawłowski A, Nowecki Z, Debiec-Rychter M, Grzesiakowska U, et al. Surgical resection of residual disease in initially inoperable imatinib-resistant/intolerant gastrointestinal stromal tumor treated with sunitinib. Eur J Surg Oncol. 2009; 35:87–91.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download