Abstract
Purpose
This study evaluated the efficacy for preventing venous thromboembolism (VTE) and adverse effects of low-molecular-weight heparin (LMWH) in order to launch a prospective clinical trial in Korea.
Methods
We reviewed the medical records of 108 consecutive patients who underwent gastric cancer surgery. These patients were divided into 2 groups according to the type of thromboprophylaxis: group A, LMWH combined with intermittent pneumatic compression (IPC); group B, IPC alone. The postoperative outcomes of the two groups were compared.
Results
Symptomatic VTE was observed in only 1 patient (0.9%) from group B. Postoperative bleeding was more common in group A than in group B (10.9% vs. 7.5%), although the difference was not significant (P = 0.055). Most bleeding episodes were minor and managed conservatively without intervention. Only a high body mass index was associated with a significantly increased risk of postoperative bleeding (odds ratio, 1.45; 95% confidence interval, 1.12-2.43; P = 0.051).
Venous thromboembolism (VTE) is related to various risk factors such as cancer, peripheral vascular disease, heart disease, immobile condition, and recent history of major abdominal or orthopedic surgery [1,2]. Among these, the presence of malignant disease significantly increases the risk of developing VTE. Therefore, hospitalized cancer patients undergoing chemotherapy or surgery appear to have the greatest risk of developing VTE [3]. In particular, patients with gastric, pancreatic, hematologic, and ovarian cancers have the greatest risks of developing VTE. Gastric cancer is associated with the fifth-highest rate of VTE [4]. A retrospective cohort study reports that the rate of VTE is 7.4% among patients with gastric cancers [5]. The American Society of Clinical Oncology VTE Guideline Panel recommends that physicians consider pharmacological thromboprophylaxis in patients undergoing major surgery for malignant disease [6]. Low-molecular-weight heparin (LMWH) has become a standard pharmacological agent for preventing VTE because it is easy to use, just once a day injection schedule.
Little study has published about the LMWH prophylaxis on patients with cancer surgery. Jeong et al. [7] reported that pharmacological thromboprophylaxis with LMWH is associated with a significant risk of bleeding complications.
The present study analyzed outcomes of LMWH prophylaxis during the gastric cancer surgery, and tried to compare with the outcomes of intermittent pneumatic compression (IPC) device without LMWH.
From July to October 2011, we reviewed 108 patients' medical records; diagnosed with histologically confirmed primary gastric adenocarcinoma, and who showed no evidence of distant metastasis upon preoperative evaluation. The patients divided into two groups: LMWH + IPC (group A) and IPC alone (group B). Use of enoxaparin depended on the surgeon's preference. We had two gastric surgeons in our hospital during this study, and only one surgeon (K.Y.S.) used enoxaparin. The Institutional Review Board of Seoul St. Mary's Hospital approved this study.
All patients had curative intent (R0) gastrectomy with extragastric lymph node dissection. We screened all patients' coagulation profiles, including bleeding time, prothrombin time, activated partial thromboplastin time, and platelet count. In order to screen the disease conditions related to hypercoagulability or bleeding tendency, the levels of protein C, protein S, antithrombin, homocysteine, factor Va, and antiphospholipid IgG/IgM were determined. All patients were managed by the critical pathway protocol; based on this, patients were encouraged to walk early on postoperative day 1 and resume diet no later than postoperative day 3.
All patients wore an IPC device to prevent VTE. IPC device was mandatory before going to the operation room and until discharge. A 40 mg of enoxaparin sodium (Clexane, Sanofi-Aventis Ltd., Seoul, Korea) was administered to patients if they were allotted to group A. Enoxaparin was injected subcutaneously at least 12 hours before surgery and continued once daily until discharge. A serum D-dimer assay was performed on postoperative days 1 and 3 to rule out VTE. Duplex ultrasonography or embolism computed tomography (CT) scan performed if there was any clinical suspicion of deep vein thrombosis or pulmonary embolism (PE).
For both groups, we prospectively collected data regarding surgical outcomes including morbidity and mortality, and hospital courses in our data-recording system.
Regarding bleeding indices, luminal bleeding was diagnosed when there was melena or hematochezia accompanied by a decrease in serum hemoglobin levels (≥2 g/dL over 24 hours) or by endoscopic findings. Intra-abdominal bleeding was suspicious, when there was bloody drainage with significant hemoglobin changes, and required radiologic confirmation; such as CT or ultrasonography. Regarding demographic characteristics, the following parameters; such as age, sex, body mass index (BMI), and comorbid medical conditions were compared. For surgical outcomes, local and systemic complications, hospital stays, postoperative fever, and diet resumption time were compared between the two groups. Postoperative complications graded by the Clavien-Dindo Classification system [8].
All statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). The χ2 test or Fisher exact test and independent 2-tailed t-tests were used to compare the clinicopathological parameters and surgical outcomes between groups A and B, wherever appropriate. Continuous variables were stratified and analyzed as categorical data. Therefore, univariate analysis was performed using the χ2 test or Fisher exact test to determine the associations between variables and bleeding complications. Moreover, backward stepwise multivariate logistic regression analysis incorporating all variables in the univariate analysis was performed. The level of statistical significance was set at P < 0.05.
There were 73 male and 35 female patients; their mean age was 57.1 ± 10.9 years. There were no significant differences between the groups with respect to age, gender, BMI, operation type, or medical comorbidities (Table 1).
Operative results showed that 81 of the subjects (75%) underwent subtotal gastrectomy, 27 (25%) underwent total gastrectomy, and 71 (65.7%) underwent laparoscopic gastrectomy. Out of 108 patients, 66 (61.1%) underwent D2 lymph node dissection with a mean operation time of 156.5 ± 45.5 minutes. No significant intergroup difference was found with respect to the type of resection, surgical approach, lymph node dissection, or operation time. Stage distribution did not differ significantly between groups.
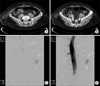
During the postoperative period, 1 female patient from group B presented with symptomatic VTE on postoperative day 14; she underwent curative subtotal gastrectomy using an open approach and experienced sudden swelling with pain in her left lower calf. CT venography revealed massive thrombotic occlusion in her left femoral vein. We performed mechanical thrombolysis followed by heparinization and inferior vena cava filter placement (Fig. 1).
In this study, 33 complications occurred in 27 patients. According to the Clavien-Dindo classification, 15 and 2 complications were grade II and IIIa, respectively.
Postoperative complication rates tended to be higher in group A than that in group B, but were not statistically significant (30.9% vs. 18.9%, P = 0.091) (Table 2). Although the incidences of intra-abdominal and luminal bleeding after operation were not significantly different between groups (P > 0.05), skin hematoma was significantly higher in group A than that in group B (group A vs. group B, 7.2% vs. 0%; P < 0.05).
Univariate analysis revealed that LMWH usage and high BMI significantly increased the risk of bleeding complications (P = 0.047 and P = 0.035, respectively). After using multivariate analysis, these factors were not significant risk factors of postoperative bleedings. The BMI showed tendency of increasing risk of postoperative bleeding (odds ratio, 1.45; 95% confidence interval, 1.12-2.43; P = 0.051) (Table 3).
The American College of Chest Physicians guidelines for thromboprophylaxis classify cancer surgery as a "high-risk" procedure for the development of VTE and recommend active prophylaxis including LMWH or unfractionated heparin [9]. The estimated incidences of deep vein thrombosis (DVT) and fatal PE in this group are 20%-40% and 0.4%-1.0% respectively [6,9]. However, in Korea, the routine use of LMWH to prevent VTE in preoperative periods was not common. Surgeons are afraid of risks of unexpected intraoperative or postoperative bleeding. Many Korean surgeons are more concerned about the postoperative bleeding than prevention of rare risk of VTE with LMWH, compared with the high incidence of VTE in the western countries [10,11].
The rate of thromboprophylaxis with LMWH is significantly lower in Asian countries than that in the Western countries because the true incidence and risks of VTE after cancer surgery in Asian patients remain uncertain. The incidence of VTE in Asian patients was approximately 3- to 5-fold lower in a previous report, although the incidence of asymptomatic VTE may be higher without thromboprophylaxis. An increased risk of postoperative bleeding is another issues related to LMWH usage.
Meta-analyses and randomized controlled trials conducted in the West revealed little or no increase in the rates of clinically significant postoperative bleeding with the use of prophylactic doses of LMWH [12-15]. However, few studies have evaluated the feasibility of LMWH prophylaxis in Asia. We are planning to determine the optimal thromboprophylaxis method during gastric cancer surgery. Before starting a prospective clinical trial, we tried to evaluate the safety of using LMWH in the present study. The LMWH tended to increase the risk of postoperative bleeding in patients with gastric cancer in our patients. However, most of the bleeding complications were minor events unrelated to mortality and mostly managed conservatively.
The surgical procedures and extent of surgery might influence bleeding risks, such as open versus laparoscopy and extent of lymph node dissection. Extensive lymph node dissection, which could increase the risk of bleeding complications, is a routine procedure for radical gastrectomy in Korea in contrast to surgery in the West [16]. Nonetheless, there was no significant difference in bleeding episodes with respect to the resection type, extent of lymph node dissection, or type of surgical approach (i.e., laparoscopic and open) between groups. Only high BMI was an independent risk factor for an increased risk of bleeding, probably because the surgical plane is more difficult to find and more friable for dissection in obese patients [17].
In order to examine the hypercoagulability status of Korean patients, we measured protein C, protein S, antithrombin, homocysteine, factor Va, and antiphospholipid IgG/IgM levels, which are well-known factors related to hypercoagulability. Interestingly, most factors were within normal limits, implying that hereditary causes of coagulopathy are not prominent among Korean patients. On postoperative day 1, 50 patients (46.2%) exhibited elevated D-dimer levels; of these patients, 3 (2.7%) who presented with asymmetric leg swelling underwent duplex ultrasonography to rule out DVT. None of these patients showed DVT.
The risk of bleeding associated with LMWH usage is hypothetically dependent on several factors including LMWH dosage and injection timing. A previous intervention study comparing the administration of 2,500 and 5,000 U LMWH in general surgical patients revealed that the incidence of DVT was significantly lower in those administered 5,000 U; however, the incidence of bleeding was significantly higher in these patients than that in patients administered 2,500 U [18]. In the present study, we used 40 mg of enoxaparin sodium, which recommended dosage in the previous trials [19,20]. We just followed this dosage, which is sufficient to prevent VTE and to have reasonable rate of bleeding complications in Korean patients, but in future trial will also confirm it.
In this study, LMWH seems to increase bleeding risks, but it does not significantly alter the patients' clinical course. LMWH seemed to have a tendency to increase the risks of bleeding in patients with high BMI.
In Asia, due to the low-reported incidence of VTE, surgeons in Asia rather not interested in VTE prophylaxis as in Western surgeons. However, the patients' population were aged and got overweight. The real incidence of VTE or LMWH related risks of bleeding were uncertain.
Based on this study, we will launch a prospective randomized clinical trial to investigate the clinical efficacy of LMWH thromboprophylaxis by comparing LMWH plus IPC prophylaxis with IPC alone in Korean gastric cancer patients after surgery.
Figures and Tables
Fig. 1
Computed tomography image revealed extensive deep vein thrombosis along the left iliac and femoral veins (A & B). An inferior vena cava filter was in serted (C & D).
References
1. Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, et al. Prevention of venous thromboembolism. Chest. 2001; 119:1 Suppl. 132S–175S.
2. Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991; 151:933–938.
3. Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006; 119:60–68.
4. Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: risk analysis using Medicare claims data. Medicine (Baltimore). 1999; 78:285–291.
5. Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006; 24:484–490.
6. Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007; 25:5490–5505.
7. Jeong O, Ryu SY, Park YK, Kim YJ. The effect of low molecular weight heparin thromboprophylaxis on bleeding complications after gastric cancer surgery. Ann Surg Oncol. 2010; 17:2363–2369.
8. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
9. Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126:3 Suppl. 338S–400S.
10. Liew NC, Moissinac K, Gul Y. Postoperative venous thromboembolism in Asia: a critical appraisal of its incidence. Asian J Surg. 2003; 26:154–158.
11. Kang MJ, Ryoo BY, Ryu MH, Koo DH, Chang HM, Lee JL, et al. Venous thromboembolism (VTE) in patients with advanced gastric cancer: an Asian experience. Eur J Cancer. 2012; 48:492–500.
12. Brown A. Preventing venous thromboembolism in hospitalized patients with cancer: improving compliance with clinical practice guidelines. Am J Health Syst Pharm. 2012; 69:469–481.
13. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients: results of meta-analysis. Ann Surg. 1988; 208:227–240.
14. Kakkar VV, Cohen AT, Edmonson RA, Phillips MJ, Cooper DJ, Das SK, et al. The Thromboprophylaxis Collaborative Group. Low molecular weight versus standard heparin for prevention of venous thromboembolism after major abdominal surgery. Lancet. 1993; 341:259–265.
15. Nurmohamed MT, Verhaeghe R, Haas S, Iriarte JA, Vogel G, van Rij AM, et al. A comparative trial of a low molecular weight heparin (enoxaparin) versus standard heparin for the prophylaxis of postoperative deep vein thrombosis in general surgery. Am J Surg. 1995; 169:567–571.
16. Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005; 92:1099–1102.
17. Kim KH, Kim MC, Jung GJ, Kim HH. The impact of obesity on LADG for early gastric cancer. Gastric Cancer. 2006; 9:303–307.
18. Bergqvist D, Burmark US, Flordal PA, Frisell J, Hallbook T, Hedberg M, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995; 82:496–501.
19. Lechler E, Schramm W, Flosbach CW. The Prime Study Group. The venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). Haemostasis. 1996; 26:Suppl 2. 49–56.
20. Sherman DG, Albers GW, Bladin C, Fieschi C, Gabbai AA, Kase CS, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007; 369:1347–1355.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download