Abstract
Endotension is an unpredictable late complication of endovascular aortic aneurysm repair (EVAR). This case report will discuss the successful treatment of enlarged aneurysmal sac due to endotension using the relining technique. An 81-year-old male complained of nondecreasing huge aneurysm sac. He had undergone EVAR for infrarenal abdominal aortic aneurysm 7 years prior and no endoleak was found through follow-up. Initially computed tomography-guided sac aspiration was tried, but in vain, Relining using the double barrel technique and tubular endograft for modular diconnection, which was unexpectedly found in the original endograft, were performed sucessfully. During follow-up after the relining procedure, the size of aneurysm sac continued to decrease in size. The relining technique is effective mothod for treating endotension.
Endovascular aortic aneurysm repair (EVAR) has become an effective first-line treatment option in high-risk patients with comorbities [1,2]. However, for patients with growing aneurysm after EVAR, additional intervention is required for the treatment of endoleak induced sac enlargement [3,4]. Recent meta-analysis reported that 16.5% of patients required secondary surgical or percutaneous interventions after EVAR because of early or late complications [5]. In rare cases of growing aneurysm sac without endoleak, the pressure within the aneurysm sac is considered as a cause. This pressure induced growing aneurysm is called endotension. But treatment to eliminate endotension has yet to be established. This case reports the successful treatment of enlarged aneurysm sac due to endotension after EVAR using the endograft relining technique.
An eighty-one-year-old male visited the clinic complaining of abdominal pain along with pulsating mass. He had been diagnosed with infrarenal aortic aneurysm and this was treated with EVAR 6 years previously. After EVAR, a gradual increasing of the aneurysm sac was found without evidence of any type of endoleak. Eventually, the aneurysm reached a diameter of 9.7 cm on the computed tomography (CT) angiography and abdominal pain redeveloped. CT angiography showed a huge aortic aneurysm without evidence of endoleak, and it was considered that this was due to endotension.
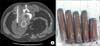
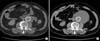
CT-guided sac aspiration was performed through the translumbar puncture of the aneurysm sac, and 400 mL of a dark brown-colored liquid was aspirated (Fig. 1). CT scan was performed the day after the puncture and the sac shrunk back to its original size (Fig. 2A). Abdominal pain subsided and the palpating mass disappeared. One month after the aspiration, a CT scan was retaken, and the scan showed that the aneurysm sac increased back to the size before the aspiration (Fig. 2B). Additionally, the scan showed no signs of endoleak. A second aspiration was performed again, and a further 300 mL of the dark brown colored fluid was aspirated. During the second aspiration, small amounts of contrast media was administered through the lumbar puncture site in order to search for the presence of collateral flow induced endoleak and to detect any possible rupture of the aneurysm sac. But no contrast media leakage from the sac was found, and no endoleak was found. So it was concluded that the increase of the aneurismal sac was due to endotension.
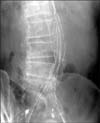
Finally, with the consent of the patient, the endovascular relining technique was performed on the patient to treat the endotension. Since the diameter of the main body of the original endograft was 28 mm, it was planned that the relining process would be carried out by inserting two customized, tubular stent-grafts each of 14 mm in diameter. The length of the relining stent-graft was calculated to cover not only the whole length of the original stent-graft but to cover the entire growing sac. The patient was put under general anesthesia, and both common femoral arteries were punctured and selection of both limbs of the original stent-graft was attempted. Unexpectedly, the guide wire escaped from the junction area of the left module to the aneurysm sac. Upon inspection, a modular disconnection was found at the left graft junction area resulting in large amounts of leakage from the disconnected area. The original main body was selected with difficulty, and two 14-mm- × 12-cm-sized stent-grafts were successfully implanted within the original endograft (Fig. 3). Ancillary attachment between the original and the new stent-grafts was made with a compliant balloon. A final aortogram was performed and no endoleak was detected and the leakage from the modular disconnection disappeared after the endovascular relining. The day after the procedure, CT angiography showed no endoleak but retroperitoneal hematoma around the aneurysm sac was found. It was determined that the contained aneurismal sac rupture and the retroperitoneal hematoma were caused by the leakage from the modular disconnection.
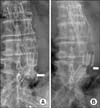
Serial review of the plain x-rays taken since the initial EVAR 6 years prior showed very subtle but gradual modular migration unnoticeable by eye, and complete disconnection developed within 5 days between the day of the second aspiration and the relining procedure. It was determined that the modular disconnection was a result of the aspiration (Fig. 4). The patient showed no symptoms or signs of aneurysm sac rupture after the procedure and was discharged with no further complications. CT scan taken four months after the relining procedure showed no endoleak and the size of the aneurysm sac decreased from 9.7 to 9.3 cm.
A successful endovascular treatment of abdominal aortic aneurysms is defined as a reduction in the size of the aneurysm sac. An increasing aneurysm sac not only means that the treatment has failed but also could lead to an endoleak induced aneurismal sac rupture. In the rare case of growing aneurysm sac without endoleak, the pressure within the aneurysm sac is considered as one of the causes, which is called endotension (endoleak type V). The incidence rate of endotension after EVAR has been reported to be about 1.5% to 5%. The origin of endotension is thought to be from unrecognized endoleak, or transudate migration through the endograft wall due to the endograft's porosity [6,7]. As a result, the growth of the aneurysm sac may extend to the landing zone that can cause endograft migration or disintegration promoting type I or type III endoleak. Because increased aneurysm sac is related to aneurysm sac rupture, patients with growing sac must be carefully treated. However, the standard treatment modality of endotension between surgical conversion and endovascular treatment is not yet established. On one hand, some authors recommend just follow-up for patients showing no symptoms because it is possible that aggressive treatment may lead to eventual and iatrogenic sac rupture.
Many endovascular treatment methods exist including graft relining of proximal and distal cuff, aspiration of the aneurysm sac, and direct percutaneous sac injection of materials like thrombin and fibrin glue. Different methods of managing endotension after EVAR have been reported in a number of case reports. Filippi et al. [8] reported that patients with increasing aneurysm sac treated with the relining technique maintained patency and sac size reduction during the three-year follow-up. Cerna et al. [9] suggest aspiration of the aneurysm sac could be an easy and effective treatment modality for endotension. Although only 3 patients were treated with this method and the follow-up was carried out for just 2 years, no subsequent enlargement nor any complication was seen in patients treated with aspiration. Uthoff et al. [10] reported an 88.9% (16 of 18 patients) success rate with the direct percutaneous sac injection method with reintervention being required for six patients within a median of 10 months after the initial injection. However, the standard treatment modality of endovascular treatment replacing surgical options is still a controversy and the best modality is yet to be determined.
In this case report, a CT-guided sac aspiration was performed initially. However, the aneurysm sac was refilled only days after the aspiration. Eventually, endograft disintegration (modular disconnection) developed and contained aneurysm sac rupture due to type 3 endoleak resulted. The endograft relining technique was immediately planned and performed which was fortunate for the patient as the modular disconnection was unnoticeable before the relining procedure. Patients who undergo EVAR are usually monitored with CT angiography scan in order to detect possible complications such as endoleak, endograft migration, and endotension.
Continual surveillance of endograft through plain x-ray is a very simple method, but easy to overlook. In a retrospective review of this case, it is possible that the migration of the stent, although barely noticeable, could be detected with continual and careful observation of plain x-ray films. Hence, the importance of serial x-ray surveillance is stressed again. We recommend plain x-ray follow-up along with CT angiography as this may provide important information. Furthermore, endograft relining techniques could be a very reliable and useful option to manage endotension induced growing aortic aneurysm sac.
Figures and Tables
Fig. 1
(A) Computed tomography (CT) angiography showing increased size of aneurymal sac (9.7 cm) without endoleak (B) Aspirate of aneurysmal sac content by CT guidance.
References
1. Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg. 2009; 50:880–896.
2. Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011; 41:Suppl 1. S1–S58.
3. Zarins CK, White RA, Hodgson KJ, Schwarten D, Fogarty TJ. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg. 2000; 32:90–107.
4. Gelfand DV, White GH, Wilson SE. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg. 2006; 20:69–74.
5. Hobo R, Buth J. EUROSTAR collaborators. Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. J Vasc Surg. 2006; 43:896–902.
6. Gilling-Smith G, Brennan J, Harris P, Bakran A, Gould D, McWilliams R. Endotension after endovascular aneurysm repair: definition, classification, and strategies for surveillance and intervention. J Endovasc Surg. 1999; 6:305–307.
7. Goodney PP, Fillinger MF. The effect of endograft relining on sac expansion after endovascular aneurysm repair with the original-permeability Gore Excluder abdominal aortic aneurysm endoprosthesis. J Vasc Surg. 2007; 45:686–693.
8. Filippi F, Tirotti C, Stella N, Rizzo L, Taurino M. Endotension-related aortic sac rupture treated by endograft relining. Vascular. 2013; 21:113–115.
9. Cerna M, Kocher M, Utikal P, Bachleda P. Endotension after endovascular treatment of abdominal aortic aneurysm: percutaneous treatment. J Vasc Surg. 2009; 50:648–651.
10. Uthoff H, Katzen BT, Gandhi R, Pena CS, Benenati JF, Geisbusch P. Direct percutaneous sac injection for postoperative endoleak treatment after endovascular aortic aneurysm repair. J Vasc Surg. 2012; 56:965–972.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download