Abstract
Purpose
To analyze the incidence and patterns of calcification of papillary thyroid microcarcinoma (PTMC) on neck ultrasonography (NUS) and assess the clinical implications of calcification, especially for neck node metastasis.
Methods
The clinical data of 379 patients with PTMC who underwent thyroidectomy between January and December 2011 were retrospectively analyzed. PTMC lesions were classified into four subgroups according to their calcification patterns on preoperative NUS: microcalcification, macrocalcification, rim calcification, and noncalcification. The clinicopathologic characteristics were compared between the patients with and without calcification, and among the four subgroups.
Results
Calcifications were detected on NUS in 203 patients (53.5%) and central neck node metastasis was observed in 119 patients (31.3%). Calcification was associated with larger tumor size (0.68 cm vs. 0.54 cm), higher rate of lymph node metastasis (38.6% vs. 23.2%) and higher lymph node ratio (0.11 vs. 0.06) compared to noncalcification (All P < 0.05). In addition, the extent of calcification correlated with lesion size (0.67 cm vs. 0.69 cm vs. 0.85 cm). Further, the likelihood of lymph node metastasis also correlated with the extent of calcification in the order of non-, micro- and macrocalcification (23.3%, 36.8%, and 44.1%, respectively). The calcification rate was higher in patients with lymph node metastasis than those without it (65.5% vs.47.7%) (All P < 0.05).
The prevalence of papillary thyroid microcarcinoma (PTMC) has increased recently, due in part to the increased use of neck ultrasonography (NUS) and NUS-guided fine needle aspiration cytology [1]. NUS features that suggest malignancy in a thyroid nodule include microcalcifications, the absence of "halo" sign, marked hypoechogenicity, extrathyroidal extension, an irregular or microlobulated margin, and a heterogeneous echo structure [2]. Calcifications on thyroid ultrasonography can be classified as micro- or macrocalcifications, and microcalcification considered to be the most specific sonographic indicator in the diagnosis of papillary thyroid carcinoma (PTC) [3].
PTCs are the most common type of thyroid cancer, often forming concentric calcified foci, called psammoma bodies, which are strongly diagnostic for PTC [4]. On NUS, psammoma bodies appear as fine, scattered, and punctate bright echoes, indicative of microcalcifications. Other types of calcification, including coarse, macro, eggshell and rim calcifications, were formerly thought to be more common in benign thyroid tumors than in malignant thyroid tumors. However, these patterns have also been observed in malignant lesions, although microcalcifications remain the most frequent type in thyroid malignancies [5].
The mechanism of formation of microcalcifications in PTC was thought to involve a poor blood supply to the nipple, leading to calcification necrosis [6]. More recently, however, osteopontin (OPN) became known as the cause of formation of microcalcifications [7]. Moreover, OPN expression was found to be significantly related to lymph node metastasis [8,9].
Thus, to date, the molecular mechanism responsible for calcification in PTC has not been determined, nor has the clinical significance of calcification in thyroid malignancy, including PTMCs. This study was therefore designed to analyze the incidence and patterns of PTMC calcification on NUS and to assess the clinical implications of PTMC calcification.
The NUS findings and clinical data of 379 patients with PTMC who underwent thyroidectomy between January and December 2011 were retrospectively reviewed. All preoperative NUS examinations were performed by one endocrine surgeon and several thyroid radiologists at Gachon University Gil Medical Center. Electronic patient records were reviewed, and NUS findings were reviewed and analyzed in detail by a single endocrine surgeon, who paid close attention to the presence and patterns of calcifications.
PTMC calcifications were classified by size and pattern as, namely, microcalcification (defined as punctate echogenic foci ≤1 mm with or without posterior shadowing), macrocalcifications (defined as punctate echogenic foci >1 mm in size), or rim calcifications (defined as nodules with peripheral curvilinear or eggshell calcifications) [10]. Of the 379 patients, 136 (35.9%) had microcalcifications, 59 (15.6%) had macrocalcification, 8 (2.1%) had rim calcifications, and remaining 176 patients (46.4%) did not have any calcifications (Fig. 1).
A total thyroidectomy with bilateral central neck compartment dissection was performed on 198 of the 379 patients, and 181 underwent unilateral lobectomy including the isthmus with unilateral central neck node dissection. Moreover, 56 patients (14.7%) who had suspicious lymph node enlargement on NUS underwent therapeutic central lymph node dissection (CND), with the remaining 323 (85.2%) undergoing prophylactic CND.
The study protocol was approved by our Institutional Review Board, which waived the requirement for informed consent due to the retrospective nature of this study.
The chi-square and independent t-tests were used to compare clinicopathologic data between the 203 patients with calcification and the 176 without calcification. Data in the four patient subgroups were compared using the analysis of variance and Kruskal-Wallis H test. All statistical analyses were performed using the IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
The mean age of the 379 patients was 48.5 years (range, 20-77 years), and the male to female ratio was 1:7 (49:330). One hundred nineteen patients (31.3%) had neck node metastasis, including 112 (94.1%) with central and 7 (5.9%) with central and lateral neck node metastasis. Patients with lymph node metastasis (LN+) were younger than those without lymph node metastasis (LN-). Lesion size (0.7 cm vs. 0.58 cm, P < 0.001), frequency of extrathyroidal extension (53.8% vs. 34.6%, P < 0.001) and multicentricity (37% vs. 26.6%, P = 0.001) were significantly higher in the LN+ than in the LN- group. In addition, the average number of retrieved central neck lymph nodes was significantly higher in LN+ than in LN- patients who underwent total thyroidectomy (17.5 vs. 11.2, P < 0.001), but not in patients who underwent lobectomy (Table 1).
Among the 119 patients with lymph node metastasis, 112 (94.1%) had only central neck node metastasis, whereas 7 (5.9%) had both lateral and central neck node metastasis. The mean number of metastatic nodes was 2.4 in patients who underwent lobectomy and 3.7 in patients who underwent total thyroidectomy (Table 2).
Calcifications on preoperative NUS were detected in 203 of the 379 patients (53.5%). The mean size of the dominant lesion was slightly larger in patients with calcification than without calcification (0.68 cm vs. 0.54 cm, P < 0.001). The percentage of patients with lymph node metastasis (38.4% vs. 23.2%, P = 0.001), the mean number of retrieved nodes (12.5 vs. 9.5, P = 0.002), the mean number of metastatic nodes (1.4 vs. 0.5, P < 0.001), and the lymph node ratio (0.11 vs. 0.06, P = 0.002) were significantly higher in patients with calcification than without calcification (Table 3). Further analysis of the patients with calcification showed a correlation between older age and larger calcification size. Mean lesion size (0.67 cm vs. 0.69 cm vs. 0.85 cm, P < 0.001) increased significantly with increasing degree of calcification, in the order of microcalcification, macrocalcification and rim calcification. The rate of lymph node metastasis (23.3% vs. 36.8% vs. 44.1%, P = 0.013) increased significantly with increasing extent of calcification, in the order of non-calcification, micro-, macrocalcification except rim calcification (25%) (Table 4). The overall calcification rate was significantly higher in patients with node metastasis than without node metastasis (65.5% vs. 47.7% P = 0.001).
The analysis of patients with node metastasis showed that the number of retrieved neck nodes was significantly higher in the group with calcification than without calcification (15.9 vs. 12.7, P = 0.001), but there were no differences in the mean number of metastatic lymph nodes and lymph node ratio among the 4 groups (Table 5).
Microcalcification on NUS is the most specific indicator in the sonographic diagnosis of PTC [3]. The incidence of calcification in thyroid malignancy has been reported in several studies. One study found that 62% of patients with microcalcifications had malignant lesions, whereas 38% were diagnosed with benign thyroid pathology [5]. Another study reported that calcifications were present in 58.1% of thyroid malignancies, with 44.2% having microcalcifications, 9.7% having macrocalcifications and 4.2% having rim calcifications [10]. A further study reported that the presence of microcalcifications in a predominantly solid nodule increased a patient's cancer risk about 3-fold [11]. Microcalcifications in thyroid lesions have been shown to have a high predictive value (42%-94%), but a low sensitivity rate (26%-59%) in the diagnosis of malignancy [3,12-14].
Most previous studies regarding calcifications in thyroid cancer were limited to nodules larger than 1 cm. A study comparing the sensitivity and specificity of microcalcification in large and small nodules, with a cutoff size of 1 cm, discovered that microcalcification was more diagnostic for thyroid cancer in nodules larger than 1 cm (51.4% sensitivity and 91.6% specificity) than in nodules smaller than 1 cm (36.6% sensitivity and 87.9% specificity) [10]. These findings suggested that the frequency of microcalcification was lower in PTMCs than in PTCs and furthermore, microcalcification was not a major predictor of malignancy in nodules ≤1 cm [10]. Therefore, we attempted to find not only the incidence and the pattern of calcification, but also clinical significance of calcification in PTMC through this study.
In our study, in which all patients had PTMC, the incidence of calcification was 53.5% (203/379), whereas the incidence of microcalcification was 35.9% (136/379). Similar results have been reported in other studies. A study showed that 58.3% of patients with PTMC (14/24) had calcified nodules and 20.8% (5/24) had fine stippled psammomatous calcifications [14]. And another study reported microcalcifications in 68 of 127 (53.5%) patients with PTMC [15].
Other types of calcifications, including macrocalcifications and eggshell or rim calcifications, were thought to be more common in benign lesions than in malignant ones. Benign nodules usually have coarse calcifications, particularly with long disease duration [16]. However, calcification patterns other than microcalcifications have also been observed in malignant lesions. For example, one study reported that macrocalcifications were equally distributed between benign and malignant lesions, with 66.7% of patients having both micro- and macrocalcifications diagnosed with cancer [5]. Although a specific type of rim calcification has been reported as indicative of malignancy [17], the relationship between rim calcification and malignancy has not yet been determined [11]. Of our 379 patients with PTMC, 8 (2.1%) had rim calcification in this study.
Histopathologically, thyroid calcifications can be classified as psammoma bodies and dystrophic calcifications, or as psammoma bodies, stromal calcifications and bone formation [18]. Psammoma bodies are laminated, basophilic, spherical accretions and are characteristic of papillary carcinomas, although they are occasionally observed in benign thyroid lesions [6,19,20].
The mechanism responsible for the formation of calcifications has not yet been fully elucidated. The rapid proliferation of cancer cells may lead to cancer tissue hyperplasia and hyperplasia mixed with necrosis, resulting in the deposit of calcium and calcifications. Therefore, psammoma bodies in PTC are found in association with tumor cells within lymphatic spaces or within the tumor stroma [6,14,21]. Alternatively, psammoma bodies may be formed by intracellular calcifications in viable cells, such as the nidus. Calcifications in PTC may not necessarily develop in nonviable and dying cells [7].
Little is known about the molecular interactions that result in psammoma bodies, stromal calcification or bone formation in the thyroid, except for the involvement of OPN, which is a member of the bone morphogenetic protein family [22]. OPN has been reported with roles related to initiation, progression and transplantation of malignant tumor in breast tumor and PTC [8,23-27]. OPN has not been detected in normal thyroid tissue [9] and further, OPN mRNA-expressing cells have been observed around psammoma bodies [8]. Moreover, OPN upregulation correlates with aggressive clinicopathological features of PTC. Hence, the presence of lymph node metastases and tumor size both positively correlated with OPN positivity [28].
Discovering that OPN expression correlates with both the formation of microcalcifications and adverse prognostic factors in PTC suggests that calcifications in PTMC may also be associated with adverse prognostic factors, including tumor size and lymph node metastasis. The presence of psammoma bodies was found to correlate significantly with gross lymph node metastasis, persistent disease on follow-up examination, higher incidence of pulmonary metastasis and poorer disease-free survival, suggesting that the presence of psammoma bodies may be a useful predictor of outcome in patients with PTC [18,29].
To date, however, few reports have assessed the clinical significance of calcifications in PTC, particularly in PTMC. Therefore, the present study focused primarily on the significance of calcifications in PTMC, discovering that patients with calcifications have a significantly higher number of metastatic neck nodes (1.4 vs. 0.5, P < 0.001) and a higher lymph node ratio (0.11 vs. 0.06, P = 0.002) compared to patients without calcifications. In addition, an analysis of patients with node metastasis demonstrated that the number of metastatic neck nodes (3.8 vs. 2.2, P = 0.119) and the lymph node ratio (0.3 vs. 0.25, P = 0.194) was higher in patients with calcification than without calcification even though there was no statistical significance. These results suggest that the presence of calcification on NUS may be predictive of neck lymph node metastasis in PTMC patients. Furthermore, the analysis of patients with a calcification group according to calcification patterns indicated a correlation between larger calcification size and higher rate of lymph node metastasis in the order of non-calcification, microcalcification, and macrocalcification except rim calcification (23.3% vs. 36.8% vs. 44.1%, P = 0.008). The reason for the discrepancy in the rim calcification may result from too small number of patients (only eight), and suggesting the need to assess a larger number of patients in order to determine the characteristics of lesions with rim calcification.
The most important limitation of this study is that we were not able to confirm the direct relationship between the role of OPN and histologic aggressiveness of PTMC, particularly with regard to neck node metastasis. Further study will be required to make such confirmations.
In conclusion, patients with PTMC having calcifications on NUS had larger tumor size, a higher rate of lymph node metastasis, and a higher lymph node ratio compared to patients without calcification. Further, the rate of lymph node metastasis increased in the order of non-, micro-, and macrocalcification. Calcification patterns should be carefully assessed by preoperative NUS in patients with PTMC. In particular, those having calcifications should undergo a thorough central neck node dissection.
Figures and Tables
Fig. 1
Papillary thyroid microcarcinoma calcification patterns on neck neck ultrasonography. (A) Noncalcification: taller than wide, ill-defined hypoechoic nodule. (B) Microcalcification: several calcifications measuring less than 1 mm. (C) Macrocalcification: one calcification larger than 1 mm. (D) Rim calcification: ring-shaped calcification on the periphery of the nodule.
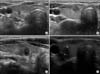
Table 1
Clinicopathologic characteristics of papillary thyroid microcarcinoma patients with and without lymph node metastasis

Table 2
Clinicopathologic characteristics of the 119 papillary thyroid microcarcinoma patients with cervical lymph node metastasis
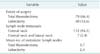
Table 3
Clinicopathologic characteristics of papillary thyroid microcarcinoma patients with and without calcification

References
1. Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003; 13:381–387.
2. Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, et al. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg. 2001; 136:334–337.
3. Khoo ML, Asa SL, Witterick IJ, Freeman JL. Thyroid calcification and its association with thyroid carcinoma. Head Neck. 2002; 24:651–655.
4. Takashima S, Fukuda H, Nomura N, Kishimoto H, Kim T, Kobayashi T. Thyroid nodules: re-evaluation with ultrasound. J Clin Ultrasound. 1995; 23:179–184.
5. Seiberling KA, Dutra JC, Grant T, Bajramovic S. Role of intrathyroidal calcifications detected on ultrasound as a marker of malignancy. Laryngoscope. 2004; 114:1753–1757.
6. Johannessen JV, Sobrinho-Simoes M. The origin and significance of thyroid psammoma bodies. Lab Invest. 1980; 43:287–296.
7. Das DK, Sheikh ZA, George SS, Al-Baquer T, Francis IM. Papillary thyroid carcinoma: evidence for intracytoplasmic formation of precursor substance for calcification and its release from well-preserved neoplastic cells. Diagn Cytopathol. 2008; 36:809–812.
8. Tunio GM, Hirota S, Nomura S, Kitamura Y. Possible relation of osteopontin to development of psammoma bodies in human papillary thyroid cancer. Arch Pathol Lab Med. 1998; 122:1087–1090.
9. Sun Y, Fang S, Dong H, Zhao C, Yang Z, Li P, et al. Correlation between osteopontin messenger RNA expression and microcalcification shown on sonography in papillary thyroid carcinoma. J Ultrasound Med. 2011; 30:765–771.
10. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008; 247:762–770.
11. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005; 237:794–800.
12. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002; 87:1941–1946.
13. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002; 178:687–691.
14. Wang N, Xu Y, Ge C, Guo R, Guo K. Association of sonographically detected calcification with thyroid carcinoma. Head Neck. 2006; 28:1077–1083.
15. Wang Y, Li L, Wang YX, Feng XL, Zhao F, Zou SM, et al. Ultrasound findings of papillary thyroid microcarcinoma: a review of 113 consecutive cases with histopathologic correlation. Ultrasound Med Biol. 2012; 38:1681–1688.
16. Kuma K, Matsuzuka F, Kobayashi A, Hirai K, Morita S, Miyauchi A, et al. Outcome of long standing solitary thyroid nodules. World J Surg. 1992; 16:583–587.
17. Kwak MS, Baek JH, Kim YS, Jeong HJ. Patterns and significance of peripheral calcifications of thyroid tumors seen on ultrasound. J Korean Radiol Soc. 2005; 53:401–405.
18. Bai Y, Zhou G, Nakamura M, Ozaki T, Mori I, Taniguchi E, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod Pathol. 2009; 22:887–894.
19. Triggiani V, Guastamacchia E, Licchelli B, Tafaro E. Microcalcifications and psammoma bodies in thyroid tumors. Thyroid. 2008; 18:1017–1018.
20. Ellison E, Lapuerta P, Martin SE. Psammoma bodies in fine-needle aspirates of the thyroid: predictive value for papillary carcinoma. Cancer. 1998; 84:169–175.
21. Klinck GH, Winship T. Psammoma bodies and thyroid cancer. Cancer. 1959; 12:656–662.
22. Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007; 26:508–523.
23. Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979; 16:885–893.
24. Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986; 83:8819–8823.
25. Sharp JA, Sung V, Slavin J, Thompson EW, Henderson MA. Tumor cells are the source of osteopontin and bone sialoprotein expression in human breast cancer. Lab Invest. 1999; 79:869–877.
26. Hirota S, Ito A, Nagoshi J, Takeda M, Kurata A, Takatsuka Y, et al. Expression of bone matrix protein messenger ribonucleic acids in human breast cancers. Possible involvement of osteopontin in development of calcifying foci. Lab Invest. 1995; 72:64–69.
27. Bellahcène A, Castronovo V. Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases. Bull Cancer. 1997; 84:17–24.
28. Guarino V, Faviana P, Salvatore G, Castellone MD, Cirafici AM, De Falco V, et al. Osteopontin is overexpressed in human papillary thyroid carcinomas and enhances thyroid carcinoma cell invasiveness. J Clin Endocrinol Metab. 2005; 90:5270–5278.
29. Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer. 1985; 55:805–828.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download