Abstract
Median arcuate ligament syndrome is a rare cause of abdominal pain which results from compression of the celiac artery (CA) or rarely, the superior mesenteric artery by a ligament formed by the right and left crura of the diaphragm. We report a case of open surgical decompression of the CA by division of the median arcuate ligament for a 37-year-old female patient who had suffered from chronic postprandial epigastric pain and severe weight loss. We described clinical features, characteristic angiographic findings and details of the surgical procedure for the patient with this rare vascular problem.
Median arcuate ligament syndrome (MALS, also called celiac axis compression syndrome or Dunbar syndrome) is known to be caused by compression of celiac artery (CA) by a fibrous arch that originates from the diaphragmatic crura on either side of the aortic hiatus and passes superior to the origin of the celiac axis. Though it is still debated whether the compression of the CA can cause chronic mesenteric ischemia or not, there have been anecdotal case reports of surgical decompression of CA by dividing the medial arcuate ligament (MAL).
The common clinical features of MALS are chronic postprandial abdominal pain characteristically augmented by full expiration, nausea or vomiting, weight loss and audible epigastric bruit. The diagnosis of MALS usually depends on the clinical features and radiologic finding of the focal narrowing at the proximal celiac axis on a lateral view of conventional aortography or computed tomography (CT).
While open surgical release of MAL has been used for the treatment of this syndrome, laparoscopic release has been reported recently [1]. Percutaneous transluminal angioplasty has also been attempted [2]. However, it was usually ineffective owing to the refractory extrinsic compression of the CA by the tight ligament structure. We would like to report a case of surgical treatment of MALS describing its clinical features, characteristic findings of diagnostic imaging study, and details of our surgical procedure.
A 37-year-old female patient presented with chronic epigastric pain lasting 6 months, food phobia and weight loss of 10 kg during the previous 4 months. The pain was cramping aggravated after meals, and persisted for 20-30 minutes after meals. There were no other gastrointestinal symptoms such as diarrhea, constipation, gastrointestinal bleeding and nausea or vomiting.
As past history, she underwent coil embolization of the bilateral ovarian veins at another hospital 2 months before visiting us under the impression of pelvic congestion syndrome, which did not improve her abdominal symptoms.
Physical examination showed mild abdominal tenderness at both lower quadrants without muscle rigidity. Laboratory test showed normal range including serum amylase, liver enzyme and complete blood cell count. There was also no abnormality in fluorescent antinuclear antibody and antineutrophil cytoplasm antibody. Gastro-duodenoscopy showed chronic atrophic gastritis and 18-fluorodeoxyglucose positron emission tomography-CT showed no spe cific lesion with abnormal hot uptake.
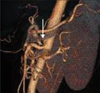
Abdominal CT angiography (Fig. 1) showed downward angulation of CA and superior mesenteric artery (SMA) close to their origins by the compression.
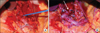
To decompress CA, we approached through the upper mid line incision and lesser sac. We found CA was tightly compressed by MAL with poststenotic dilatation (Fig. 2). After division of the fibrous structure, we found that arterial blood flow increased significantly and recovered without bruit.
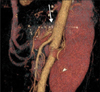
Postoperatively, epigastric postprandial pain was resolved and the patient could return to a normal diet. On a follow-up abdominal CT angiography (Fig. 3), we found patent CA with mild residual stenosis.
MAL was first described by Lipshutz [3] as an anatomic structure that caused CA compression in 1917. Thereafter, Harjola [4] and Dunbar et al. [5] described it as a clinical syndrome causing nausea, vomiting and postprandial pain in 1963 and 1965.
The origin of CA and MAL varies in its location from T11 to L1, and occasionally, their locations are in conflict with each other when MAL extends inferiorly or CA originates su periorly. During deep inspiration in the erect position, CA descends to caudal direction, so the compression is relieved. But during expiration, the condition is opposite, and compression causes the symptom and is thus called MALS.
The pain mechanism is currently in debate. Postprandial pain and weight loss is associated with chronic mesenteric ischemia. But pathognomic features of CA compression on expiration cannot explain clinical improvement by revascularization. At this point, it is proposed that MALS is related with neurogenic pain from the compression and intermittent ischemia of splanchnic nerve plexus [6]. This pain can be caused either by nerve stimulation leading to vasoconstriction or by direct sympathetic fiber irritation. Splanchnic nerve plexus is an autonomic nerve plexus supplying upper abdominal organs (stomach, liver, gallbladder, pancreas). This is located in front of diaphragmatic crura around the origin of CA and SMA [7].
To make a diagnosis of MALS, other common causes of abdominal pain should be ruled out. Routine laboratory blood tests including amylase, lipase and tumor markers for hidden malignancy, esophago-gastro-duodenoscopy, liver, pancreas and kidney ultrasonography are usually used. Gold standard diagnostic modality was in lateral view of aortic angiogram. A typical feature is focal narrowing of CA with poststenotic dilatation aggravated during deep inspiration. Nowadays, 3-dimentional reconstructed CT angiography has replaced the role of conventional aortography [1].
Standard treatment of MALS is an open surgical division of MAL followed by a dramatic symptom relief. Recently, a lapa roscopic approach can be attempted but carries the risk of arterial injury and massive hemorrhage. Three series of case reports showed mean rates of open conversion due to bleeding at about 20%, but despite that, laparoscopic treatment showed no other morbidity or mortality and shortened hospital stay. There were no differences in symptom recurrence rates between laparoscopic and open surgical decompression [8]. The role of balloon angioplasty is under debate. However, current data do not support the use of balloon expandable stents [9]. This is probably due to the extraluminal compression by MAL, which should be solved outside of the CA. In cases of recurrent symptom after surgical decompression of CA, angioplasty is beneficial [10].
Figures and Tables
Fig. 1
Computed tomographic angiography: celiac trunk stenosis. Compression of celiac artery (CA) makes an acute angle of CA (arrow).
References
1. Duffy AJ, Panait L, Eisenberg D, Bell RL, Roberts KE, Sumpio B. Management of median arcuate ligament syndrome: a new paradigm. Ann Vasc Surg. 2009. 23:778–784.
2. Saddekni S, Sniderman KW, Hilton S, Sos TA. Percutaneous transluminal angioplasty of nonatherosclerotic lesions. AJR Am J Roentgenol. 1980. 135:975–982.
3. Lipshutz B. A composite study of the coeliac axis artery. Ann Surg. 1917. 65:159–169.
4. Harjola PT. A rare obstruction of the coeliac artery: report of a case. Ann Chir Gynaecol Fenn. 1963. 52:547–550.
5. Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 1965. 95:731–744.
6. Skeik N, Cooper LT, Duncan AA, Jabr FI. Median arcuate ligament syndrome: a nonvascular, vascular diagnosis. Vasc Endovascular Surg. 2011. 45:433–437.
7. Petrella S, Prates JC. Celiac trunk compression syndrome: a review. Int J Morphol. 2006. 24:429–436.
8. Tulloch AW, Jimenez JC, Lawrence PF, Dutson EP, Moore WS, Rigberg DA, et al. Laparoscopic versus open celiac ganglionectomy in patients with median arcuate ligament syndrome. J Vasc Surg. 2010. 52:1283–1289.
9. Gloviczki P, Duncan AA. Treatment of celiac artery compression syndrome: does it really exist? Perspect Vasc Surg Endovasc Ther. 2007. 19:259–263.
10. Cinà CS, Safar H. Successful treatment of recurrent celiac axis compression syndrome: a case report. Panminerva Med. 2002. 44:69–72.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download