Abstract
Inflammatory myofibroblastic tumor (IMT) of the liver is a very rare lesion that has radiologic similarity with malignant liver tumor. Differential diagnosis of IMT from a malignant lesion of the liver is very important because surgical resection is not mandatory for IMT. Lipiodol computed tomography is a very sensitive and specific diagnostic tool for hepatocellular carcinomas (HCC). Herein, we describe a case of IMT that had dense lipiodol uptake in the tumor and mimicked HCC. To our knowledge, previously, only one case of IMT with dense lipiodol retention has been reported.
Inflammatory myofibroblastic tumor (IMT) of the liver is a very rare lesion, benign in nature [1]. IMT has also been called inflammatory pseudotumor (IPT). Differential diagnosis of IMT from malignant lesions of the liver is very important because surgical resection is not mandatory for IMT and the prognosis is very good by conservative treatment [2]. But, preoperative diagnosis is very difficult due to its radiologic similarity with intrahepatic cholangiocarcinoma, metastatic tumor and hepatocellular carcinoma (HCC) [2,3].
Lipiodol computed tomography (CT), which is checked following lipiodol infusion via the hepatic artery, is a very sensitive and specific imaging modality for HCC [4]. Herein, we describe a case of IMT that had dense lipiodol uptake in the tumor mimicking HCC.
A 60-year-old woman was admitted at our hospital for evaluation and management of liver tumor. Two months ago, she felt general weakness. She had weight loss, 5 kg/2 wk. She visited a local hospital due to the above problems. She was checked by abdominal sono whereby a liver tumor was identified. Laboratory results at that time were not available. The impression was HCC at that time. Thus, she was treated with transhepatic arterial chemoembolization (TACE) by using adriamycin (50 mg), lipiodol and gelfoam. Two weeks after TACE, she visited our hospital for operation. She felt general weakness and had no other symptoms. She had diabetes mellitus that was treated with oral hypoglycemic agents. She had no history of hepatitis B or C. Blood pressure was 120/70 mmHg. Pulse rate was 76/min. Temperature was 36.0℃. She was not icteric. Neither abdominal mass nor liver were palpable.
Abnormal laboratory results were decreased hemoglobin, 10.0 g/dL (reference range, 12.0 to 16.0 g/dL); elevated erythrocyte sedimentation rate, 122 mm/hr (reference range, 0 to 22 mm/hr); and elevated gamma-glutamic transpeptidase, 81 IU/L (reference range, 7 to 32 IU/L). Hepatitis B surface antigen was negative and surface antibody was positive. Hepatitis C antibody was negative. Tumor markers were all nor mal; alpha-fetoprotein, 3.2 ng/mL (reference range, 0 to 10 ng/mL); carcinoembryonic antigen, 0.4 ng/mL (reference range, 0 to 5 ng/mL); carbohydrate antigen 19-9, 10.2 U/mL (reference range, 0 to 37 U/mL).
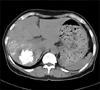
Abdomen CT checked prior to TACE showed a gross 5 cm spherical tumor in Segment VII (Couinaud's segment) and Segment VI of the liver. The tumor showed slight low attenuation on precontrast scan (Fig. 1A). On arterial phase, this tumor showed general high attenuation with central stellate slight low attenuation (Fig. 1B). On portal phase, a major portion of the tumor showed remarkable low attenuation with irregular septa-like and peripheral thick rim-like high enhancement (Fig. 1C). Hepatic artery angiogram showed spherical tumor stain with no remarkable hypervascularity (Fig. 1D). Lipiodol CT, which was taken 14 days following TACE, revealed a 4.3 cm densely lipiodol uptaken spherical mass in segment S6 and S7 of the liver (Fig. 2). Right post sectionectomy was done with the diagnosis of HCC.
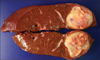
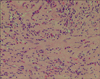
In the specimen, the tumor was 5.2 cm×4.5 cm×4.2 cm in size. It was spherical, well-defined and had smooth contour without capsule. The color of tumor was grayish white to yellow tan (Fig. 3). High power view demonstrates interlacing bundles of myofibroblasts and fibroblasts in a collagenous stroma, with an admixture of polymorphic inflammatory cells, including plasma cells, lymphocytes and eosinophils (Fig. 4). Immunohistochemical stain revealed positive activity of vimentin (diffuse +), smooth muscle actin (mainly +) and desmin (focal +). The final diagnosis was IMT.
The patient recovered well without any complication. Regular follow-up studies including abdomen CT showed no recurrence of tumor for 5 years after operation.
IMT, which also has been called IPT, is characterized by a mixture of myofibroblasts, fibroblasts, lymphocytes, and plasma cells in varying proportions. The designation of IMT is regarded as a more accurate term than IPT [1]. IMTs of the liver have rarely been reported, but nowadays reported cases are increasing probably due to the recent development of image modality [5]. Torzilli et al. [6] reported the incidence of IMT was 0.7% by analyzing 473 patients with focal liver lesion that had been resected and the patients with IMTs accounted for 20% of the wrong preoperative diagnoses and 33% of wrong indications for surgery. The majority of IMT can be managed successfully by medical treatment and the prognosis is very good [2]. Therefore, the accurate preoperative diagnosis of IMT is essential for avoiding unnecessary surgery, which may have some morbidity and mortality.
However, preoperative diagnosis by radiological modalities only is very difficult because there are few specific radiologic findings for IMT. Liver dynamic CT can reveal the enhancement pattern of the tumor during the different phases. Some authors reported that peripheral high enhancement on delayed phase was specific for IMT [2,5,6]. This finding can easily differentiate IMT from HCCs but not from metastatic liver tumors or cholanigiocellular carcinomas [2]. However, this point cannot be applicable to all cases of IMT. Some cases had high enhancement on arterial phase and faded out on portal and delayed phase [3]. In our case, the tumor showed general high attenuation on arterial phase and low attenuation with peripheral rim enhancement on portal phase. In such cases, HCCs cannot be excluded, exactly. There were a few cases of IMTs that had been diagnosed as HCCs preoperatively and resected [3,6,7]. For fear of tumor spillage through needle track, preoperative needle biopsy is not recommended for resectable HCC. Therefore, preoperative radiological diagnosis is most essential for the management of HCC.
Lipiodol CT checked after intra-arterial injection of iodolized oil is widely used for the detection of HCC. Ngan [8] reported when dense homogenous uptake was present in a discrete mass, the chance of it being an HCC was high with post-test probability being 92.8%. In our case, contrast enhanced CT showed a well-enhanced tumor on arterial phase and lipiodol CT revealed dense homogenous uptake of lipiodol in tumor, by which findings were most compatible with HCC. To our knowledge, previously, only one case of IMT with dense lipiodol retention has been reported [3]. In general, lipiodol CT is checked 2 to 4 weeks following intra-arterial lipiodol infusion. In normal hepatic parenchyma, lipiodol is almost completely washed out at that time. However, lipiodol in HCC tissue mostly remains and can help in detecting small HCC. The reason for the discrepancy of lipiodol retention rate in normal parenchyma and tumor tissue has not been clearly explained yet. One plausible hypothesis is that it may be caused by the difference of blood flow. The tumor vessels that are tortuous and irregular and often lack both a muscular layer and elastic lamellae do not have sufficient blood flow to clear away the adhesive iodized oil [9]. In this case, it was supposed that hepatic artery embolization for presumed HCC decreased the blood flow about the tumor and dense lipiodol remained in the tumor. The resected specimen of this case showed severe necrosis. Choi et al. [10] reported that HCC with complete intratumoral retention of lipiodol had 98% necrosis but tumors with incomplete retention had 64% necrosis.
In conclusion, radiologic findings of IMT including liver dynamic CT and lipiodol CT can mimic HCC. Therefore, if other clinical data including viral status or tumor marker are not compatible for HCC, a more aggressive diagnostic work-up should be needed.
Figures and Tables
Fig. 1
Abdomen computed tomography prior transhepatic arterial chemoembolization showed gross 5 cm spherical tumor in Segment VII (Couinaud's segment) and Segment VI of liver. Tumor showed slight low attenuation on precontrast scan (A), general high enhancement with central stellate slight low attenuation on arterial phase (B) and remarkable low attenuation with irregular septa-like and peripheral thick rim-like high enhancement on portal phase (C). Hepatic artery angiogram showed spherical tumor stain with no remarkable hypervascularity (D).
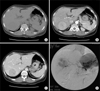
Fig. 2
Lipiodol computed tomography revealed 4.3 cm densely lipiodol uptaken spherical mass in Segment VII and Segment VI of liver.
References
1. Dehner LP. The enigmatic inflammatory pseudotumours: the current state of our understanding, or misunderstanding. J Pathol. 2000. 192:277–279.
2. Yamaguchi J, Sakamoto Y, Sano T, Shimada K, Kosuge T. Spontaneous regression of inflammatory pseudotumor of the liver:report of three cases. Surg Today. 2007. 37:525–529.
3. Tsou YK, Lin CJ, Liu NJ, Lin CC, Lin CH, Lin SM. Inflammatory pseudotumor of the liver: report of eight cases, including three unusual cases, and a literature review. J Gastroenterol Hepatol. 2007. 22:2143–2147.
4. Zheng XH, Guan YS, Zhou XP, Huang J, Sun L, Li X, et al. Detection of hypervascular hepatocellular carcinoma: Comparison of multi-detector CT with digital subtraction angiography and Lipiodol CT. World J Gastroenterol. 2005. 11:200–203.
5. Fukuya T, Honda H, Matsumata T, Kawanami T, Shimoda Y, Muranaka T, et al. Diagnosis of inflammatory pseudotumor of the liver: value of CT. AJR Am J Roentgenol. 1994. 163:1087–1091.
6. Torzilli G, Inoue K, Midorikawa Y, Hui AM, Takayama T, Makuuchi M. Inflammatory pseudotumors of the liver: prevalence and clinical impact in surgical patients. Hepatogastroenterology. 2001. 48:1118–1123.
7. Park KS, Jang BK, Chung WJ, Cho KB, Hwang JS, Kang YN, et al. Inflammatory pseudotumor of liver: a clinical review of 15 cases. Korean J Hepatol. 2006. 12:429–438.
8. Ngan H. Lipiodol computerized tomography: how sensitive and specific is the technique in the diagnosis of hepatocellular carcinoma? Br J Radiol. 1990. 63:771–775.
9. Nakakuma K, Tashiro S, Hiraoka T, Uemura K, Konno T, Miyauchi Y, et al. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer. 1983. 52:2193–2200.
10. Choi BI, Kim HC, Han JK, Park JH, Kim YI, Kim ST, et al. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992. 182:709–713.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download