Abstract
Purpose
Although spontaneous intramural hematomas of the gastrointestinal tract are very rare, they may be observed with the use of oral anticoagulant, though less frequently in cases of hematological malignancy and other bleeding disorders. Cases diagnosed as spontaneous intramural hematoma have been assessed in our clinic.
Methods
The cases, which were diagnosed as spontaneous intramural hematoma in the gastrointestinal tract (SIHGT) following anamnesis, physical examination, biochemical, radiological and endoscopic findings from July 2008 to July 2012, have been assessed retrospectively.
Results
Seven out of 13 cases were women and the mean age was 65.1 years (34 to 82 years). The most frequent complaint on admission was abdominal pain. The most frequent location of SIHGT was the ileum (n = 8). Oral anticoagulant use was the most common cause of etiology (n = 12). In 10 cases, International normalized ratio values were higher than treatment range (2 to 3, where mechanical valve replacement was 2.5 to 3.5) and mean value was 7.6 (1.70 to 23.13). While 12 cases were discharged without problems with medical treatment, one case with acute myeloid leukemia died in the intensive care unit following cerebrovascular attack.
Conclusion
Spontaneus bleeding and hematomas that may arise in connection with bleeding diathesis may be fatal in cases with long-term oral anticoagulant treatment and insufficient follow-up. In management of these cases, it may be necessary to arrange conservative follow up and/or initialize low molecular weight heparin, and administer vitamin K as well as replace blood products and coagulation factors when indicated.
Spontaneous intramural hematomas of the gastrointestinal tract (SIHGT) is a relatively rare but a well-known clinical entity. The pathophysiological mechanism of SIHGT is intramural bleeding due to the shredding of terminal arteries at the point where they leave the mesentery and penetrate the muscular layer of the bowel wall [1,2]. The bleeding is usually self-limited. However, if bleeding continues, subsequent intramural hematoma can dissect through a plain between submucosal and muscular layers, and thereby may involve a long segment of the bowel [3,4]. Intramural hematoma eventually obstructs the intestinal lumen, which results in a partial or complete mechanical bowel obstruction [1,2,5]. Moreover, intramural hematoma alters the intramural osmotic gradient, which in turn leads to intraperitoneal hemorrhagic effusion [6,7].
The main predisposing factor for the development of SIHGT is any type of anticoagulant therapy [1,8,9], which is followed by hematological diseases including malignancies and bleeding disorders. Therefore, differential diagnosis should include SIHGT in any patients with the risk factors mentioned above who present with acute abdominal pain or mechanical bowel obstruction with or without gastrointestinal bleeding [8,10].
Preoperative diagnosis of SIHGT is crucial for avoiding un ne cessary surgical exploration because the treatment of SIHGT is conservative in the vast majority of cases [11-13]. However, the radiological findings should be interpreted cautiously because, in abdominal ultrasound (US) and computed tomography (CT) screenings, tubular or circular images with lack of peristalsis, mucosal compressions surrounded by an anechoic halo [14], thickened bowel wall, partial or complete obstruction of the bowel lumen, pseudo-kidney or coiling spring signs and short segment dilatation of flap [5,12] are nonspesific and may also be observed in inflammatory, ischemic and neoplastic conditions [10]. Therefore, diagnostic tools are valuable if only combined with a high index of clinical suspicion for SIHGT.
In this retrospective analysis, we aimed to assess the manage ment and the outcome of patients with SIHGT in terms of oral anticoagulant (OAC) usage and the transfusion of blood products protocols.
The study was designed as a retrospective analysis. Medical records of 21 patients with abdominal pain and a high inter national normalized ratio (INR) value were reviewed. Seven patients were excluded due to hematoma of the rectus abdominis and one patient was excluded due to a prior surgery for mesenteric ischemia. Thirteen patients who were diagnosed with SIHGT between July 2008 and July 2012 were analyzed.
All patients were analyzed for demographics, coexisting medical conditions, clinical presentations, laboratory and radiological imaging studies in the emergency setting.
After SIHGT was diagnosed, oral intake was ceased and parenteral fluid-electrolyte replacement was initiated. Nasogastric decompression was considered only for patients with vomiting due to bowel obstruction.
Fresh frozen plasma (FFP) was more likely to be administered to cases with an extremely high INR value and who had active bleeding findings, or for which invasive procedure was planned. Erythrocyte suspension was given to the patients who had cardiac disease with a haemoglobin level below 10 g/dL. Low molecular weight heparin (LMWH) was initialized in cases with an INR value of less than 2. The patients with gastrointestinal bleeding underwent endoscopic evaluation to rule out other possible causes of bleeding such as peptic ulcer or gastritis.
The median length of hospital stay and bowel rest, morbidity and mortality rates were recorded. Results are expressed as median values with the interquartile range or mean values ± standard deviations.
The mean age and female/male ratio was 65.1 years (34 to 82 years) and 7/6, respectively. The most frequent complaint on admis sion was abdominal pain (n = 11). On admission, two cases had upper gastrointestinal (GI) bleeding and three cases had findings suggesting mechanical intestinal obstruction. Gin gival bleeding and epistaxis were present in one patient.
OAC use for cardiovascular diseases (aortic or mitral valve stenosis with valve replacement in 5 cases, ischemic heart disease with post coronary artery bypass graft in 5 cases, atrial fibrillation in one case, post pulmonary embolus with vena cava filter in one case) was the most common cause of SIHGT (n = 12). In one patient, acute myeloid leukemia (AML) caused coagulation disorder and SIHGT. The mean INR on admission was 7.6 (1.7 to 23.13). INR value was above treatment range in 10 cases. In the rest of the three cases, INR values were 3.2 (with mitral valve replacement with mechanical valve [MVR]), 2.02, 1.70, respectively.
All cases were examined with abdominal US screening upon admission (n = 13). Unenhanced abdominal CT screening was performed on one case with renal function deterioration. Contrast enhanced triphasic abdominal CT screening was performed on the rest of the cases. Findings of screening methods are summarized in Table 1.
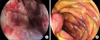
Two cases with GI bleeding and one case with uncertainty of proximal GI tract obstruction were assessed with upper GI endoscopy. Endoscopy was performed after FFP replacement in two of these cases with INR values of 3.7 and 2.6, respectively. In the cases with GI bleeding, hemorrhage and submucosal hematoma at various sites in the esophagus and stomach (Fig. 1A) and submucosal hematoma in the second portion of the duodenum with extensive erosions in the antrum (Fig. 1B) were detected, respectively. Distal duodenal and proximal jejunal submucosal hematoma without obstruction findings were detected in the third case.
The most frequent location of SIHGT was the ileum (n = 8) as detected by endoscopy and/or CT assessment. Jejunum was the second most common (n = 6) localization of involvement (isolated in three cases, with duodenum in one case and ileum in two cases).
OAC was withdrawn immediately in all patients. Oral intake was also interrupted in all patients with acute abdominal pain, GI bleeding and symptoms of bowel obstruction on admission. The mean length of bowel rest for all patients was 3.3 ± 1.23 days.
Three cases had mechanical bowel obstruction with persisting small intestine type air fluid levels in plain abdo minal radiography. In these cases, decompression was performed with nasogastric (NG) catheter with the interruption of oral intake and, afterwards, parenteral fluid was started. The mean length of bowel rest in these patients was 4.0 ± 1.73 days. NG catheter was removed when they were able to pass gas and stool and, afterwards, oral nutrition was initiated. The length of fasting was not prolonged as long as to necessitate the use of total parenteral nutrition in any patients.
FFP replacement was performed after discontinuing OAC in 10 cases. FFP replacement was carried out in 3 cases with active bleeding (upper GI endoscopy was performed in two of the three patients), five cases due to potential candidacy for abdominal emergency surgery and two cases with extremly high INR values (23.1 and 14.02). The diagnostic tools and treatment options used by our clinic are summarized in Table 2. Vitamin K was administered to 8 cases, except 5 cases with MVR (vitamin K is standard therapy except for valve replacement patients). Two cases with INR value between 2-3 and one case with mitral valve disease were monitored without replacement. These three cases' mean INR value was 3.2. All cases with INR values of under 2 were started on LMWH (n = 10).
The mean length of hospital stay was 6.4 days (2 to 13 days) and 12 were discharged following nonoperative treatment. The case that was diagnosed with AML died in the intensive care unit (Table 3). One month after discharge, eight of 12 cases were screened with CT. CT findings revealed a partial resolution of hematoma in 87.5% (n = 7). Physical examination of these eight patients were also uneventful six months after discharge. None of these patients needed further intervention or admission to hospital during this period.
While SIHGTs are generally observed after abdominal trauma, they are detected in an increasing number of cases that use OAC due to more frequent use of diagnostic imaging devices. Other possible factors can be trauma, hemophilia, von Willebrand disease, idiopathic thrombocytopenic purpura, leukemia, lymphoma, vasculitises, collagenosis, chemotherapy and bone marrow transplantation, liver diseases, peptic ulcer, protein C and protein S synthesis disorders [10,13]. Pancreas diseases (such as pancreatitis) may be both the result and the reason for SIHGTs [8,14].
OACs are among the main etiological factors in nontraumatic cases [5] and the SIHGT rate is about 1/2,500 in cases using long-term OAC [8,13]. Post-trauma SIHGT was not observed in our cases and SIHGT detection rate was 1/2,190 in all emergency consultations for the patients using OAC. One of our cases was diagnosed with AML, and subsequently with intramural hematoma during follow-up.
Frequent INR monitoring of patients who are treated with OAC therapy is required during follow-up. If levels detected are above treatment range, there is an increased risk of bleeding. However, bleeding or hematoma have been reported even in treatment range in cases using OAC [12]. SIHGT may be observed when coagulation parameters are in treatment range or close to normal values, as with the three cases using OAC in our study [1,12,15].
Since symptoms vary according to the location of hematoma in patients with SIHGT, patients may apply to emergency clinics with different clinical presentations. As in our study, lo ca lized or expanded abdominal pain, nausea, black stool, gingival bleeding, defecation difficulty and vomiting are the most frequent symptoms [8,14,16]. GI tract bleedings generally manifest in the form of melena and it is reported in about 40% of cases, which is in contrast with the rate observed in our study (15.3%) [8].
Despite diagnostic adequacy of USG, CT is more a sensi tive modality for detecting the location and volume of the bleeding [12] and highly suggestive of the presence of hematoma in almost 100% of cases [12,17,18]. Polat et al. [19] reported a definite diagnosis rate up to 100% with combined use of US and CT. In our study, US and CT were used in conjunc tion in twelve cases during diagnosis.
Small intestine involvement is >80% in SIHGTs linked to OACs, with the jejunum being the most frequently involved location [5,8]. Similarly, small intestine involvement has been detected in 92.3% of our cases, ileum being the most frequent location (61.5%).
The clinical treatment consists of OAC withdrawal. Interruption of oral intake is also essential in patients with GI bleeding, severe acute abdominal pain with rebound tenderness or nausea and vomitting. In the presence of mechanical obstruction, gastric decompression, and correction of electrolyte disturbances are necessary [8].
The use of FFP and other factor products are not recommended due to their limited effect except for cases with active bleeding [20]. Where there is no indication, usage should be avoided since transfusion related acute lung damage, graft versus host disease, venous thromboembolism, allergy, infection and other complications connected to leukocyte depletion may result in morbidity and mortality. In cases with active bleeding, 15 mL/kg FFP infusion is recommended, but the dose may be adjusted depending on the clinical course and monitoring [20].
In our study, out of 10 cases whose INR value was above the treatment range, three of them had active bleeding (2 upper GI bleeding, one epistaxis and gingival bleeding). To these three cases, FFP and vitamin K were administered at the emergency clinic at time of admission to help coagulation cascade. Vitamin K was not administered to cases with MVR.
Repeated bleeding and later period complications such as development of stenosis are very rare. One month after discharge, a control CT screening was performed in 8 of the twe lve surviving cases and SIHGT did not increase, or it decreased partially.
Surgical exploration, which was preferred more in the past, should be considered in complications such as intestinal ischemia or suspected perforation, peritonitis, intra-abdominal hemorrhage and persistent GI blockages [5,8,13]. Surgery was not needed in the management of our cases, including patients with mechanical obstruction.
In conclusion, SIHGT is one of the intraabdominal compli cat ions of anticoagulant treatment. It should be considered in the diagnosis of patients with a history of OAC use and reporting acute abdomen symptoms or mechanical bowel obstruction with or without gastrointestinal bleeding. Combined use of US and CT can provide a definitive diagnosis with almost 100% accuracy. It is necessary to withdraw medication in existence of OAC use, while simultaneously administering vitamin K if indicated, and monitoring the patient for close follow-up.
The treatment of SIHGT is conservative in many patients. In management of these cases, it may be necessary to arrange blood and blood product for cases with active bleeding or for those requiring invasive procedure. Surgical intervention should be reserved for cases with unclear diagnosis or suspected complications.
Figures and Tables
Table 1
Findings on ultrasound and computed tomography screening of patients with spontaneous intramural hematomas of the gastrointestinal tract

Table 2
Management of patients with spontaneous intramural hematomas of the gastrointestinal tract

INR, international normalized ratio; OAC, oral anticoagulant; MVR, mitral valve replacement with mechanical valve; FFP, fresh frozen plasma; LMWH, low molecular weight heparin.
a)INR values of the patients were 23.1 and 14.02. b)One of the patients was acute myeloid leukemia (INR, 1.7) with massive bleeding, the other patient had gastrointestinal bleeding, with a history of MVR and INR was 3.2 (in the tratment range but higher for an invasive procedure).
References
1. Carkman S, Ozben V, Saribeyoglu K, Somuncu E, Erguney S, Korman U, et al. Spontaneous intramural hematoma of the small intestine. Ulus Travma Acil Cerrahi Derg. 2010. 16:165–169.
2. Askey JM. Small bowel obstruction due to intramural hematoma during anticoagulant therapy, a non-surgical condition. Calif Med. 1966. 104:449–453.
3. Hale JE. Intramural intestinal haemorrhage: a complication of anticoagulant therapy. Postgrad Med J. 1975. 51:107–110.
4. Nakayama Y, Fukushima M, Sakai M, Hisano T, Nagata N, Shirahata A, et al. Intramural hematoma of the cecum as the lead point of intussusception in an elderly patient with hemophilia A: report of a case. Surg Today. 2006. 36:563–565.
5. Abbas MA, Collins JM, Olden KW. Spontaneous intramural small-bowel hematoma: imaging findings and outcome. AJR Am J Roentgenol. 2002. 179:1389–1394.
6. Judd DR, Taybi H, King H. Intramural hematoma of the small bowel; a report of two cases and a review of the literature. Arch Surg. 1964. 89:527–535.
7. Jarry J, Biscay D, Lepront D, Rullier A, Midy D. Spontaneous intra mural haematoma of the sigmoid colon causing acute intestinal obstruction in a haemophiliac: report of a case. Haemophilia. 2008. 14:383–384.
8. Sorbello MP, Utiyama EM, Parreira JG, Birolini D, Rasslan S. Spontaneous intramural small bowel hematoma induced by antico agulant therapy: review and case report. Clinics (Sao Paulo). 2007. 62:785–790.
9. Abbas MA, Collins JM, Olden KW, Kelly KA. Spontaneous intramural small-bowel hematoma: clinical presentation and long-term outcome. Arch Surg. 2002. 137:306–310.
10. Lane MJ, Katz DS, Mindelzun RE, Jeffrey RB Jr. Spontaneous intramural small bowel haemorrhage: importance of non-contrast CT. Clin Radiol. 1997. 52:378–380.
11. Shah P, Kraklow W, Lamb G. Unusual complication of coumadin toxicity. Wis Med J. 1994. 93:212–214.
12. Hatipoglu AR, Hoscoskun Z, Ahsen M. The acute abdomen due to use of oral anticoagulants: spontaneous intraabdominal hemorrhage (a case report). Ulus Travma Acil Cerrahi Derg. 1997. 3:88–90.
13. Kilbas Z, Harlak A, Ersoz N, Ozerhan IH, Mentes O, Eryilmaz M. A rare cause of acute abdomen due to warfarin toxicity: spontaneous intramural intestinal hematoma. JAEM. 2009. 8:43–45.
14. Farhoud S, Stephani SM, Bromberg SH. Acute pancreatitis due to intramural hematoma of the duodenum by the use of anticoagulants. Arq Gastroenterol. 2001. 38:53–56.
15. Uzun MA, Koksal N, Gunerhan Y, Sahin UY, Onur E, Ozkan OF. Intestinal obstruction due to spontaneous intramural hematoma of the small intestine during warfarin use: a report of two cases. Eur J Emerg Med. 2007. 14:272–273.
16. Lorente-Ramos RM, Santiago-Hernando A, Del Valle-Sanz Y, Arjonilla-Lopez A. Sonographic diagnosis of intramural duodenal hematomas. J Clin Ultrasound. 1999. 27:213–216.
17. Secil M, Ucar G. Spontaneous duodenal hematoma. J Emerg Med. 2004. 27:291–293.
18. Acea Nebril B, Taboada Filgueira L, Sanchez Gonzalez F, Freire Rodriguez D, Fraguela Marina J, Aguirrezabalaga Gon, et al. Acute abdomen in anticoagulated patients: its assessment and the surgical indications. Rev Clin Esp. 1995. 195:463–467.
19. Polat C, Dervisoglu A, Guven H, Kaya E, Malazgirt Z, Danaci M, et al. Anticoagulant-induced intramural intestinal hematoma. Am J Emerg Med. 2003. 21:208–211.
20. O'Shaughnessy DF, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004. 126:11–28.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download