Abstract
Purpose
Tissue adhesion is a well-known postsurgical phenomenon, causing pain, functional obstruction, and difficult reoperative surgery. To overcome these problems, various synthetic and natural polymer membranes have been developed as postoperative tissue adhesion barriers. However, limitation in their use has hindered its actual application. We prepared a hyaluronate membrane (HM) to evaluate its efficacy and safety as an adhesion barrier compared to a commercialized product (Interceed, Ethicon).
Methods
To evaluate the antiadhesion effect, a cecum-abdominal wall abrasion model was adopted in a rabbit. The denuded cecum was covered by Interceed or HM or neither and apposed to the abdominal wall (each, n = 10). Four weeks after surgery, the level of adhesion was graded. Acute and chronic toxicity of the three groups were also evaluated.
Results
Blood samples drawn to evaluate acute toxicity at postoperative day 3 and 7 showed no significant difference among the three groups. The grade and area of adhesion were significantly lower in the HM compared to those of the control and Interceed at four weeks after surgery. Histologic evaluations, which was carried out to estimate tissue reactions at the site of application, as well as to assess chronic toxicity for the major organs, were not significantly different in the three groups.
Postoperative tissue adhesion is still a major cause of postoperative complications, such as bowel obstruction [1], chronic pain [2], and infertility [3]. If symptoms persist without improvement through conservative treatment, surgical intervention should be considered [4], which may give difficulties to both surgeons and patients in surgery. Efforts to prevent adhesion formation include minimal dissection during operation, use of anti-inflammatory drugs or tissue plasminogen activators preventing fibrin formation [5] or use of antiadhesive agents acting as a physical barrier [6].
Antiadhesive agent can be applied at the site where adhesion might occur and remain as a physical barrier for the time being. It should also be degraded and absorbed by the body after the critical period at which adhesion may develop, not to remain as a foreign material.
The main ingredient for antiadhesive agents is biocompatible polymers, which include oxidized regenerated cellulose, dextran, carboxymethylcellulose (CMC), hyaluronic acid (HA) in polysaccharides and polyethylene glycol, poloxamer, poly (lactic acid), poly (glycolic acid), poly (lactic co-glycolic acid), and Gore-tex in synthetic polymers [7].
The representative antiadhesive agents are Interceed (Ethicon, Somerville, NJ, USA) [8-10], which was synthesized from ORC; Seprafilm (Genzyme Co., Cambridge, MA, USA) [9,10] crosslinked HA and CMC; and Surgiwrap (Mast Biosurgery, San Diego, CA, USA) [11,12] using Poly (lactic acid).
Although the above tissue adhesion barriers can be applied in a situation when difficulty arises in use of solution or gel type tissue adhesion barriers, low effectiveness and safety hin ders wide use of the agents. Therefore, a new antiadhesive agent is in need of development.
The antiadhesion agent used in this study is a membrane type product composed of HA. Mechanical textures were improved in terms of flexibility, absorption time and adhesiveness to tissues compared to conventional product.
In this study, the efficacy and safety of hyaluronate membrane (HM) tissue adhesion barrier was evaluated through a rabbit cecum-abdominal wall adhesion model.
The HM was manufactured by crosslinking Sodium Hyaluronate
(HA, Shisheido Co., Tokyo, Japan) with 1,4 butanediol
diglycidyl ether (BDDE, Sigma-Aldrich Co., St. Louis, MO,
USA). It has uinform pores sized 10.0 ± 3.7 µm on scanning
electron microscopy (Fig. 1).
This animal study was authorized with Institutional Animal Care and Use Committees of Genewel Co. (approved no. Bio-111010-01). Animals for experiments were maintained in compliance with all regulatory guidelines by Korea Ministry of Food and Drug Safety.
Three to four month-old female New Zealand white rabbits weighing 2.5-3 kg were purchased from DBL Co. (Eumseong, Korea). All the animals were stabilized for 1 week before the surgical manipulation. Open abdominal surgery was performed to make models for intra-abdominal wall and cecum abrasion.
To induce general anesthesia, Zoletil 50 (0.2 mL/kg, Virbac, Carros cedex, France) and Rompun (0.03 mL/kg, Bayer Korea, Seoul, Korea) were used. The animals induced with general anesthesia were placed in the supine position, shaved in the abdominal area, prepared with povidone solution, and then draped in a sterile manner. Five to six centimeters of an anterior midline incision was made through the abdominal wall and peritoneum. After identifying the cecum, the serosal surface of the cecum was abraded by bone burr until an area of 3 cm2 × 3 cm2 was denuded as evidenced by punctuate bleeding without hemostasis. The opposing peritoneal surface of the abdominal wall was also abraded by bone burr until the same size area deperitonized. Animals were grouped into three categories; negative control group, positive control group (Interceed) and experimental group (HM) (each, n = 10). A 4 cm × 4 cm tissue adhesion barrier was applied to cover the entire area exposed and fixed with nonabsorbable sutures with 5/0 nylon sutures (AILee Co., Busan, Korea).
The abdominal incision was closed with 4/0 Nylon suture, skin was closed with 3/0 Nylon suture and 330 mg D-pron (Handong Co., Seoul, Korea) and 8.5 mg gentamycin (Deasung Microbiological Institute, Seoul, Korea) were injected into thigh muscles. All the animals were fed with sufficient food and water, and they were checked daily for any abnormal signs and symptoms.
Four weeks after the operation, the animals were sacrificed, and the adhesion formation was evaluated according to adhesion scoring system as follows by a blinded and inde pendent staff using the data: score 0, no adhesion; score 1, filmy adhesion easily separable with blunt dissection; score 2, mild to moderate adhesion with free dissection; score 3, moderate to dense adhesions with difficult dissection or nondissection [13,14] (Fig. 2).
Additionally, the adhesion area was measured, and adhesion area was expressed as mean ± standard deviation. Statistical difference in the adhesion scores and areas from the three groups was made by analysis of variance (ANOVA) test. A P-value < 0.05 was considered statistically significant.
To evaluate acute toxicity of the applied antiadhesive agents, 5 mL blood samples were drawn from the ear vein in each ani mal on postoperative day 3 and 7. The blood samples were tested for the following categories: glutamic oxaloacetic transa minase, glutamic pyruvic transaminase, creatinine, blood urea nitrogen, red blood cell (RBC), white blood cell (WBC), hematocrit (Hct), and hemoglobin (Hb).
To investigate inflammation response and the degree of tissue healing, the injured tissues including the abdominal wall and cecum were excised and fixed in 10% formaldehyde for 24 hours. After dehydration in graded series of ethanol, the specimens were embedded in paraffin wax and cut into 5-µm transverse section in the center of the injury site. These sections were stained with hematoxylin and eosin (H&E) for observation by light microscopy. The morphometric evaluation consisted of a quantitative analysis of inflammatory response by the counting of leukocytes. The number of cells on each slide (observation area, 425 µm2 × 425 µm2) was counted and the content of leukocytes was calculated as follows: Percentage of leukocyte (%) = (no. of leukocytes/no. of total cells) × 100. The granulation tissue formation around injured tissue was also blindly evaluated according to the following grading system: 0, no granulation tissue; 1, focal proliferation of granulation. Tissue less than on low-power field; 2, intermediate degree of granulation tissue between 1 and 3; 3, diffuse proliferation of granulation tissue involving more than half thickness of the abdominal muscle [15]. For the statistical treatment, ANOVA test was used and it was considered statistically significant at P < 0.05. Additionally, to evaluate organ toxicity, liver, kidney and spleen were procured from the animals, H&E staining was executed and they were observed by light microscopy [16,17].
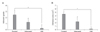
To test the safety of the applied antiadhesive agents, blood samples obtained from each group on postoperative days 3 and 7 were analyzed for liver and kidney function. The result indicated no significant difference among the three groups (Fig. 3). RBC, WBC, Hct and Hb also showed no significant difference among the three groups (Fig. 4).
The animals were sacrificed at the 4th week after the opera tion and the adhesion severity was evaluated. Within a day, an animal from negative control group and another animal from positive control group died to make the final samples as 9 in negative control group, 9 in positive control group, and 10 in experimental group. The cause of mortality of control group was suggested as an anesthesia-related problem.
In the adhesion grade evaluation, negative control group showed 2.44 ± 0.72; positive control group (Interceed), 1.6 ± 0.51; and experimental group (HM), 0.22 ± 0.66. There was statistically significant difference between the three groups (P < 0.05) (Fig. 5A). Additionally, in adhesion area, negative control group showed 4.5 ± 0.73 cm2, positive control group 2.87 ± 1.24 cm2, and experimental group 0.22 ± 0.66 cm2, which confirmed the significant reduction of adhesion area in experimental group (P < 0.05) (Fig. 5B). From these results, it was confirmed that HM had better adhesion-prevention effects than Interceed.
The inflammatory response was evaluated as the ratio of the number of leukocyte cells over total cells (%). All three groups such as negative control group, positive control group (Interceed), and experimental group (HM) showed similar re sults and there was no statistically significant difference (P = 0.507). It was confirmed that HM did not have any safety problems in inflammatory response results. There was no statistically significant difference in granulation tissue formation (P = 0.641). As experimental group showed similar results as negative control group in granulation tissue formation, it was confirmed that HM did not lead to any abnormal side effects during wound healing in a rat animal model.
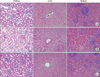
In the histological observation of liver, kidney and spleen to test the safety and toxicity against major organs, there was no infiltration of inflammatory cell or tissue necrosis in experimental group just as in negative control group (Fig. 6).
Postoperative tissue adhesion occurs in almost every part of the human body. For abdominal surgery, postoperative intraabdominal adhesion has been reported to occur at a frequency of 60-95% [6]. Although many studies are actively conducted to prevent postoperative intra-abdominal adhesion, little is known of methods to prevent this phenomenon. Generally, recom mended measures to prevent postoperative adhesion are to minimize the denuded area by careful dissection during sur gery, to activate tissue plasminogen activator or to use physical barriers.
Antiadhesive agents are applied at the site where adhesion might occur and remain for the time period while the healing process takes place, functioning as a physical barrier and preventing adhesion. It should normally degrade and absorb into the body, not to remain as a foreign material. They are available in the form of solution, gel, film or membrane.
Liquid type agent has fluidity, giving embrocating difficulty on the exact application site. It is also easily dissolved and absorbed, lowering its efficacy. Membrane or film type freely distorts its shape, and its flaccidity hinders its application through trocar in laparoscopic surgery.
Seprafilm is a semitransparent film made of HA and CMC. Its highly fragile property gives difficulty in handling during operative procedures [11,12]. Surgiwrap composed of biodegradable poly (lactic acid) is a transparent, thin film. Its hydrophobic nature does not allow effective adherence to tissue and thus requires additional suture. It may also cause inflammation by producing acid degradation during absorption period [18-20].
Another commercialized product, Interceed is easy to use in laparoscopic surgery due to its flexibility acquired from its mesh structure. However, made of oxidized cellulose, it is considered as xenobiotics and has low biocompatibility and low adhesiveness to tissues. Furthermore, when bleeding is not com pletely controlled at the applied site, its efficacy is lowered [9,10]. Accordingly, it is necessary to develop a tissue adhesion barrier that has enhanced safety, flexibility, and adhesiveness to tissues.
The HM used in this study is composed of HA, which has been considered as ideal tissue adhesion barriers due to its biocompatibility, high flexibility, and excellent adhesiveness to adjacent tissues by uniform pores on its surface. Additionally, through crosslinking with 1,4 butanediol diglycidyl ether, absorption time of HM can be controllable. Therefore, it can remain as a physical barrier during critical periods in which unwanted adhesion can form between organ or tissue.
In this study HM showed superior efficacy in terms of adhesion severity compared to Interceed. Wiseman et al.[10] demonstrated that Interceed had low efficacy in an animal model due to low tissue adhesiveness. Boland and Weigel [21] reported that blood infiltration renders the Interceed completely ineffective in preventing adhesion. Flanklin [22] showed that Interceed completely resorbed within two weeks after insertion. These findings indicate that Interceed showed low adhesion prevention effect due to low tissue adhe siveness, blood infiltration and fast degradation.
On the other hand, HM can adhere well to injured tissue via uniform pores on the surface, resulting in separation of the damaged tissue during healing process. Also, it can prevent cell or tissue invasion effectively by negative charge repulsion, which is an electronic characteristic of HA. HM showed little toxicity in the test of blood and tissue. Thus, it is confirmed that HM does not raise any safety issues by crosslinking with 1,4 butanediol diglycidyl ether.
From these results, HM can be proposed as an alternative in solving the problems of conventional membrane type tissue adhesion barriers such as difficulty in handling, lower adhesiveness to tissues, and insufficient efficacy.
Figures and Tables
Fig. 1
(A) Gross photo of hyaluronate membrane and (B) its scanning electron microscopy (×1,000). Uniform pore sized 10.0 ± 3.7 µm can be seen.

Fig. 2
Classification of adhesion severity during laparotomy at 4 weeks postsurgery. (A) Score 0, no adhesion; (B) score 1, filmy adhesion easily separable with blunt dissection; (C) score 2, mild to moderate adhesion with free dissection; (D) score 3, moderate to dense adhesions with difficult dissection or nondissection.
Fig. 3
Evaluation of analysis acute toxicity at 3 days and 7 days postsurgery. (A) Glutamic oxaloacetic transaminase, (B) glutamic pyruvic transaminase, (C) creatinine, and (D) blood urea nitrogen (*P > 0.05). HM, hyaluronate membrane.
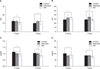
Fig. 4
Evaluation of analysis acute toxicity at 3 days and 7 days postsurgery. (A) Red blood cell, (B) white blood cell, (C) hematocrit, and (D) hemoglobin (*P > 0.05).

Fig. 6
Histological findings of kidney, liver, and spleen at 4 weeks postsurgery. (A) Control, (B) Interceed, and (C) hyaluronate membrane. Abnormal features such as inflammatory responses or tissue necrosis were not noted for three groups. Moreover, the histological observation showed no significant differences among the groups in the examined organ tissue (H&E, ×200).
References
1. Yang HY, Suh KS, Youn YK, Kim SW, Kim SJ, Lee KU, et al. A clinical analysis of intestinal obstruction in the adult. J Korean Surg Soc. 1997. 52:335–342.
2. Almeida OD Jr, Val-Gallas JM. Conscious pain mapping. J Am Assoc Gynecol Laparosc. 1997. 4:587–590.
3. Marana R, Muzii L. DiZerega GS, editor. Infertility and adhesions. Peritoneal surgery. 2000. New York: Springer-Verlag;329–333.
4. Lee WJ, Moon BI, Park JB, Kim IM, You BO. The factors influencing the treatment for the mechanical intestinal obstruction due to adhesion. J Korean Surg Soc. 1995. 49:192–203.
5. Falk K, Bjorquist P, Stromqvist M, Holmdahl L. Reduction of experimental adhesion formation by inhibition of plasminogen activator inhibitor type 1. Br J Surg. 2001. 88:286–289.
6. Shim HS, Lee YW, Lee YM, Oh YH, Kwon SW, Kim JH, et al. Evaluation of resorbable materials for preventing surgical adhesion on rat experiment. J Korean Surg Soc. 2002. 63:179–186.
7. Burns JW, Colt MJ, Burgees LS, Skinner KC. Preclinical evaluation of Seprafilm bioresorbable membrane. Eur J Surg Suppl. 1997. 40–48.
8. Haney AF, Doty E. Murine peritoneal injury and de novo adhesion formation caused by oxidized-regenerated cellulose (Interceed [TC7]) but not expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane). Fertil Steril. 1992. 57:202–208.
9. Wiseman DM, Gottlick-Iarkowski L, Kamp L. Effect of different barriers of oxidized regenerated cellulose (ORC) on cecal and sidewall adhesions in the presence and absence of bleeding. J Invest Surg. 1999. 12:141–146.
10. Wiseman DM, Trout JR, Diamond MP. The rates of adhesion development and the effects of crystalloid solutions on adhesion development in pelvic surgery. Fertil Steril. 1998. 70:702–711.
11. Rajab TK, Wallwiener M, Talukdar S, Kraemer B. Adhesionrelated complications are common, but rarely discussed in preoperative consent: a multicenter study. World J Surg. 2009. 33:748–750.
12. Song HJ, Kim JW, Park JS, Kim YS, Choi YS, Kim BG, et al. Effects of three different types of anti-adhesive agents in a rat abdominal wall defect model. J Korean Surg Soc. 2009. 77:7–14.
13. Yaacobi Y, Israel AA, Goldberg EP. Prevention of postoperative abdominal adhesions by tissue precoating with polymer solutions. J Surg Res. 1993. 55:422–426.
14. Luciano AA, Hauser KS, Benda J. Evaluation of commonly used adjuvants in the prevention of postoperative adhesions. Am J Obstet Gynecol. 1983. 146:88–92.
15. Oh SH, Kim JK, Song KS, Noh SM, Ghil SH, Yuk SH, et al. Prevention of postsurgical tissue adhesion by anti-inflammatory drug-loaded pluronic mixtures with sol-gel transition behavior. J Biomed Mater Res A. 2005. 72:306–316.
16. Lee YW, Chu B, Lee YG, Kim NH, Kim JH, Kim KI, et al. Efficacy and safety of the electrospun nanofibrous adhesion barrier for laparoscopic surgery in a rabbit model. J Korean Surg Soc. 2009. 76:73–80.
17. Kwon SW, Lim SH, Lee YW, Lee YG, Chu BY, Lee JH, et al. Anti-adhesive effect of poloxamer/alginate/CaCl2 mixture in the rat model. J Korean Surg Soc. 2006. 71:280–287.
18. Avital S, Bollinger TJ, Wilkinson JD, Marchetti F, Hellinger MD, Sands LR. Preventing intra-abdominal adhesions with polylactic acid film: an animal study. Dis Colon Rectum. 2005. 48:153–157.
19. Ersoy E, Ozturk V, Yazgan A, Ozdogan M, Gundogdu H. Effect of polylactic acid film barrier on intra-abdominal adhesion formation. J Surg Res. 2008. 147:148–152.
20. Cordewener FW, Bos RR, Rozema FR, Houtman WA. Poly(L-lactide) implants for repair of human orbital floor defects: clinical and magnetic resonance imaging evaluation of long-term results. J Oral Maxillofac Surg. 1996. 54:9–13.
21. Boland GM, Weigel RJ. Formation and prevention of postoperative abdominal adhesions. J Surg Res. 2006. 132:3–12.
22. Franklin RR. Reduction of ovarian adhesions by the use of Interceed. Ovarian Adhesion Study Group. Obstet Gynecol. 1995. 86:335–340.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download