Abstract
Purpose
Inhibition of the intimal hyperplasia after vascular surgery is an important issue. The purpose of this study is to define whether perivascular application of rapamycin, imatinib mesylate or cysteamine can reduce intimal hyperplasia in a carotid balloon injury model.
Methods
Each drug was mixed with 40% pluronic gel solution and was topically applied over the injured carotid artery evenly. Two or four weeks after injury, the arteries were harvested and morphometric analysis was done.
Results
The medial areas were not significantly different in each group and a thinning of the media as a toxic drug effect was not observed in any treatment group. The intimal area and intima-to-media (I/M) ratio were significantly reduced in rapamycin-treated group and imatinib-treated group (P < 0.05). But cysteamine-treated group showed a trend of decrease in I/M ratio in 2 weeks, but no difference in 4 weeks.
Arterial restenosis caused by intimal hyperplasia is a significant complication after both open and endovascular procedure [1,2]. Vascular injury initiates inflammatory responses with increased secretion of a number of growth factors and proteases, which results in vascular smooth muscle cell (VSMC) proliferation and migration to form a neointima with subsequent accumulation of extracellular matrix [3,4]. Many efforts were made to prevent the intimal hyperplasia, but no drug is currently available in clinical use by oral or systemic administration. Recently drug-eluting stents coated with rapamycin or paclitaxel have shown strong inhibitory effect on restenosis in patients with coronary artery stenosis [5,6]. Drug-coated balloons are now under investigation for their inhibitory effect on intimal hyperplasia (IH) after balloon angioplasty or in-stent restenosis [7]. But in open surgery, no proven therapy for preventing IH is available at the moment. Theoretically, local perivascular application of certain antiproliferative agents at the site of operative injury may be ideal for open vascular surgical patients and it is believed to work as in endoluminal drug-eluting stents. Local delivery of the drug is considered better than systemic drug administration because high effective dose delivery may be possible without severe systemic side effects.
The main issue about perivascular delivery is how to make a sustained drug release over the period of active process of cell proliferation. Drug-eluting bioabsorbable vascular wrap showed promising results in animal studies [8]. Kwon et al. [9] reported that perivascular delivery of paclitaxel with F-127 pluronic gel inhibited neointimal hyperplasia in a rat carotid artery injury model. Pluronic F-127 consists by weight of approximately 70% ethylene oxide and 30% propylene oxide with an average molecular weight of 11,500. The unique characteristics of this copolymer is reverse thermal gelation; fluid at refrigerator temperature (4℃-5℃), but are soft gels at body temperature. Pluronic F-127 could be useful as a drug vehicle for topical drug delivery system [10].
Some drugs currently used in clinic for other diseases can be applied as a novel drug to inhibit IH by perivascular delivery. Rapamycin (rapamune, Pfizer, New York, NY, USA) has dramatically reduced restenosis rates after coronary revascularization [5]. Imatinib mesylate (glivec, Novartis, Basel, Switzerland) showed inhibitory effects on IH in animal models [11]. Cysteamine is a transglutaminase inhibitor used in Huntington disease. Previously, we have shown that intimal hyperplasia was significantly attenuated in transglutaminase (TG)-null mice than in wild type mice after carotid balloon injury (on submission).
The purpose of this study is to define whether perivascular application of rapamycin, imatinib or cysteamine can reduce intimal hyperplasia in a balloon injury model of rat carotid arteries.
Male Sprague-Dawley rats weighing approximately 300 g were purchased from Koatech Inc. (Pyeongtaek, Korea), and underwent endothelial denudation of carotid artery using a balloon catheter as described elsewhere [12]. Briefly, animals were anesthetized with zoletil (Virbac, Carros, France) 10 mg/kg and xylazine (rompun, Bayer, Leverkusen, Germany) 5 mg/kg intraperitoneally. Left carotid artery was dissected via midline cervical incision. A Fogarty balloon catheter 2 Fr (Edwards Lifesciences, Irvine, CA, USA) was introduced, advanced into the common carotid artery through the external carotid artery, inflated with 0.2 mL saline, and passed 3 times in the common carotid artery. After removal of the catheter, the external carotid artery was ligated. Forty-eight rats were divided into four groups, 12 rats in each group. Rapamycin was applied in group 1, imatinib mesilate was applied in group 2, and cysteamine was applied in group 3. Group 4 was a control. The animals were allowed to recover from anesthesia, following wound closure. Access to food was not restricted except during 4 hours prior to dosing. Humane care was applied in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health [NIH] publication No. 85-23, revised 1996). The protocol for this study was approved by the Seoul National University Hospital Institutional Animal Care and Use Committee (SNUH-IACUC, No. 11-0235).
F-127 pluronic powder (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in cold distilled water to form a 40% gel solution and maintained at 4℃ for 48 hours. Cysteamine hydrochloride (Sigma-Aldrich) 1 g was dissolved in phosphate buffered saline 10 mL to make a 880 mM stock. An aliquot of 180 uL of this cysteamine stock was added to 720 uL of 40% pluronic gel solution to make 0.9 mL (60 mg/kg) mixture solution A. A total of 1-mL mixture solution A was topically applied over the injured carotid artery evenly. Rapamycin (Rapamune, 1 mg; Pfizer) was dissolved in 1-mL dimethyl sulfoxide (MERCK, Darmstadt, Germany) and 0.3 mL of this solution was added to 0.7 mL of the previously cooled 40% pluronic gel solution to make 1-mL mixture solution B containing a concentration of 1 mg/kg. A total of 1-mL mixture solution B was topically applied over the injured carotid artery evenly. Imatinib mesylate (Gleevec, Novartis, Basel, Switzerland) 100 mg/tab was dissolved in 10 mL pH 4 buffer solution (Thermo Scientific Orion pH 4.01 Buffer Solution, Thermo Fisher Scientific, Beverly, MA, USA) and 0.3 mL of this solution was added to 0.7 mL of 40% pluronic gel solution to make a concentration of 10 mg/kg BW. A total of 1-mL mixture solution C was topically applied over the injured carotid artery evenly.
Two and four weeks after drug administration, rats were anesthetized and carotid arteries were fixed by perfusion with 4% paraformaldehyde. After immersion fixation, the tissues were embedded in paraffin and sections were stained with hematoxylin-eosin. The cross-sectional areas of the arterial wall, including the lumen area, intimal area, and medial area, were quantified by using NIH Image J program (version 1.47b) and the intima-to-media (I/M) ratio were calculated. The numbers of VSMCs were also evaluated under four high power fields and used as the mean values.
The medial areas were not significantly different in each group at both time points of 2- and 4-weeks (Fig. 1). Thinning of the media can be interpreted as a toxic effect of the drug which might cause long term complications, including aneurysm formation. Such a thinning of the media was not observed in any treatment group.
The intimal area was significantly reduced in rapamycin group at 2 weeks after injury (0.06 ± 0.02 mm2 vs. 0.2 ± 0.11 mm2, P = 0.016). And in 4-week specimens, the intimal area was reduced (0.12 ± 0.07 mm2 vs. 0.21 ± 0.04 mm2, P = 0.055). The I/M ratios were significantly reduced in rapamycin group compared to control in 2-week (P = 0.025) and 4-week specimens (P = 0.033) (Fig. 2).
The intimal area and I/M ratio were reduced in imatinib group compared to control in 2-week specimens after carotid injury, but not statistically significant (0.11 ± 0.05 mm2 vs. 0.2 ± 0.11 mm2, P = 0.136) (Fig. 3). But in 4-week specimens, the intimal area and I/M ratio were significantly reduced in imatinib group compared to control (0.12 ± 0.04 mm2 vs. 0.21 ± 0.04 mm2, P = 0.027)
Two weeks after injury, the intimal area decreased in cysteamine group, but not statistically significant (0.11 ± 0.04 mm2 vs. 0.2 ± 0.11 mm2, P = 0.262) (Fig. 4). But after 4 weeks, there was no difference in the intimal area (0.23 ± 0.05 mm2 vs. 0.21 ± 0.04 mm2, P = 0.286). I/M ratio showed similar results.
In this study, we tested three promising drugs to inhibit IH; rapamycin, imatinib mesylate, and cysteamine. Perivascular delivery has several theoretical advantages. Intimal hyperplasia is a local disease where this biologic response develops as a response to injury. After open vascular procedures, such as endarterectomy, interposition graft or bypass graft, some antiproliferative agents can be applied directly at the site of operative injury. Also, the drugs can be used in high concentration without significant systemic adverse events compared to systemic use. Actually, many efforts were made to inhibit IH by perivascular local delivery of the drugs, but no drug is available in clinical use yet [8].
Rapamycin is a mammalian target of rapamycin inhibitor and mainly used in organ transplantation. It is currently used in patients with coronary artery disease by intraluminal delivery as drug-eluting stents, which has shown significant inhibitory effect on IH after balloon angioplasty or stenting [5]. Prevention of restenosis with oral rapamycin has been successful after angioplasty in animal models [13].
Imatinib mesylate is an inhibitor of the platelet-derived growth factor receptor kinase, bcr-abl kinase, and c-Kit receptor kinase [14], and is clinically used for the treatment of leukemia and gastrointestinal stromal tumor. In animal models, it has shown inhibitory effects on intimal hyperplasia [11,15], transplant arteriopathy [16], and diabetes-associated atherosclerosis [17]. Recently, Vamvakopoulos et al. [18] reported that rapamycin and imatinib mesylate successfully inhibited IH and showed synergistic effect in rat carotid injury models.
Cysteamine (β-mercaptoethylamine) is a transglutaminase inhibitor. Although cysteamine is less potent in inhibiting TG-2 than cystamine, it is the intracellular form of cystamine, which is water-soluble, that is to be used in this situation. TG-2 (TG2 or tissue-type TG) is a calcium-dependent enzyme that catalyzes transamidation reaction between glutamyl residue of protein and either lysyl residue of protein or polyamines, producing crosslinked or polyaminated proteins [19]. By these modifications of substrate proteins, TG2 is involved in apoptosis, differentiation, cell adhesion and extracellular matrix formation [20]. Previously, we have shown that transglutaminase has an important role in the process of arterial remodeling and arterial response to injury in mouse carotid injury model. Intimal hyperplasia was significantly attenuated in TG-null mice after carotid balloon injury than in wild type mice (unpublished data). With this result, inhibition of TG activity is expected to inhibit IH in this model.
In this study, perivascular application of rapamycin or imatinib mesylate reduced the intimal hyperplasia significantly, but cysteamine did not. Interestingly, cysteamine showed an inhibitory effect early after application (2-week specimen), but the effect was lost after 4 weeks. One possible explanation of this result is that the TG inhibition by cysteamine was substituted by other enzymes like coagulation factor XIII.
This study has several limitations. The duration of effective drug delivery by the perivascular gel is not defined. After 2- and 4-week periods, the local tissue concentrations of the drugs need to be investigated, but it was not performed in this study because of technical difficulties. And the submaximal dose of perivascular delivery was not defined. This is a pilot study to test the possibility of perivascular delivery of the drugs with pluronic gel, which showed promising results. Therefore, further studies with multiple drug concentrations are warranted. In this study, the medial area was not reduced in drug delivery groups. In a report of high dose paclitaxel application, the toxic effect of medial thinning and arterial ectasia was reported [21], which might cause later aneurysmal change and rupture. In this study, this kind of toxic effect on the vessel wall is not observed.
In conclusion, perivascular application of pluronic gel containing rapamycin or imatinib inhibited the development of intimal hyperplasia in rat carotid injury models. Gel with cysteamine showed early inhibitory effect, but did not inhibit the development of intimal hyperplasia at 4 weeks after injury. Further studies to define the local tissue concentration of the drugs and to adjust submaximal dose of each drug is needed.
Figures and Tables
 | Fig. 1Comparison of the medial area. The medial areas were not significantly different in each group. |
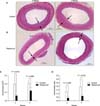 | Fig. 2Effect of rapamycin treatment. (A, B) Cross-sections of the carotid arteries in control and after local application of rapamycin. (C) Intimal area was reduced in rapamycin group. (D) Intima-to-media ratio was reduced in rapamycin group. |
References
1. Bauters C, Isner JM. The biology of restenosis. Prog Cardiovasc Dis. 1997; 40:107–116.
2. Kester M, Waybill P, Kozak M. New strategies to prevent restenosis. Am J Cardiovasc Drugs. 2001; 1:77–83.
3. Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983; 49:327–333.
4. Min SK, Kenagy RD, Clowes AW. Induction of vascular atrophy as a novel approach to treating restenosis: a review. J Vasc Surg. 2008; 47:662–670.
5. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003; 349:1315–1323.
6. Park SJ, Shim WH, Ho DS, Raizner AE, Park SW, Hong MK, et al. A paclitaxel-eluting stent for the prevention of coronary restenosis. N Engl J Med. 2003; 348:1537–1545.
7. Navarese EP, Austin D, Gurbel PA, Andreotti F, Tantry U, James S, et al. Drug-coated balloons in treatment of in-stent restenosis: a meta-analysis of randomised controlled trials. Clin Res Cardiol. 2013; 102:279–287.
8. Kohler TR, Toleikis PM, Gravett DM, Avelar RL. Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable Vascular Wrap paclitaxel-eluting mesh. J Vasc Surg. 2007; 45:1029–1037.
9. Kwon JS, Park SS, Kim YG, Son JH, Lee YS, Kim KS, et al. Perivascular delivery of paclitaxel with F-127 pluronic gel inhibits neointimal hyperplasia in a rat carotid artery injury model. Korean Circ J. 2005; 35:221–227.
10. Miyazaki S, Takeuchi S, Yokouchi C, Takada M. Pluronic F-127 gels as a vehicle for topical administration of anticancer agents. Chem Pharm Bull (Tokyo). 1984; 32:4205–4208.
11. Myllärniemi M, Frosen J, Calderon Ramirez LG, Buchdunger E, Lemstrom K, Hayry P. Selective tyrosine kinase inhibitor for the platelet-derived growth factor receptor in vitro inhibits smooth muscle cell proliferation after reinjury of arterial intima in vivo. Cardiovasc Drugs Ther. 1999; 13:159–168.
12. Min SK, Huh S, Ahn MS, Ha J, Chung JK, Kim SJ. Expression of MMPs and TIMPs in balloon-injured rat artery. Asian J Surg. 2001; 24:270–277.
13. Gregory CR, Huie P, Billingham ME, Morris RE. Rapamycin inhibits arterial intimal thickening caused by both alloimmune and mechanical injury. Its effect on cellular, growth factor, and cytokine response in injured vessels. Transplantation. 1993; 55:1409–1418.
14. Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000; 295:139–145.
15. Wang CH, Anderson N, Li SH, Szmitko PE, Cherng WJ, Fedak PW, et al. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ Res. 2006; 99:617–625.
16. Sihvola R, Koskinen P, Myllarniemi M, Loubtchenkov M, Hayry P, Buchdunger E, et al. Prevention of cardiac allograft arteriosclerosis by protein tyrosine kinase inhibitor selective for platelet-derived growth factor receptor. Circulation. 1999; 99:2295–2301.
17. Lassila M, Allen TJ, Cao Z, Thallas V, Jandeleit-Dahm KA, Candido R, et al. Imatinib attenuates diabetes-associated atherosclerosis. Arterioscler Thromb Vasc Biol. 2004; 24:935–942.
18. Vamvakopoulos JE, Petrov L, Aavik S, Lehti S, Aavik E, Hayry P. Synergistic suppression of rat neointimal hyperplasia by rapamycin and imatinib mesylate: implications for the prevention of accelerated arteriosclerosis. J Vasc Res. 2006; 43:184–192.
19. Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009; 89:991–1023.
20. Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci. 2006; 11:1057–1076.
21. Signore PE, Machan LS, Jackson JK, Burt H, Bromley P, Wilson JE, et al. Complete inhibition of intimal hyperplasia by perivascular delivery of paclitaxel in balloon-injured rat carotid arteries. J Vasc Interv Radiol. 2001; 12:79–88.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download