Abstract
Purpose
This study aimed to assess the cytolytic activity and the phenotype of circulating blood immune cells in cancer patients by using a simple preparation of peripheral blood mononuclear cells (PBMCs).
Methods
Peripheral blood was obtained from 94 diagnosed colorectal cancer (CRC) patients and 112 healthy donors. PBMCs were cocultured with K562 cells for 2 hours and lactate dehydrogenase released from the dead K562 cells was measured by using a spectrophotometer. Meanwhile, PBMCs were stained with fluorescence conjugated monoclonal antibodies (mAbs) and analyzed by flow cytometry.
Results
The cytolytic activity of PBMCs were significantly different between CRC patient and healthy groups (8.82% ± 3.84% vs. 17.51% ± 8.57%; P < 0.001). However, no significant difference in the cytolytic activity was observed after surgery in the CRC patient group (before surgery, 8.82% ± 3.84% vs. after surgery, 9.95% ± 4.94%; P = 0.326). In addition, the percentage of peripheral blood natural killer cells was significantly higher in the preoperative patient group than in the healthy group (19.97% ± 11.51% vs. 15.60% ± 5.77%, P = 0.041). In contrast, the percentage of peripheral blood lymphocytes was lower in the preoperative patient group than in the healthy group (28.41% ± 8.31% vs. 36.4% ± 8.6%, P < 0.001).
Colorectal cancer (CRC) is one of the commonly diagnosed cancers, the incidence and mortality of which are increasing worldwide every year [1]. Many research efforts have shown that it is highly correlated with various factors such as older age, gender, alcohol use, smoking, and intake of fatty foods, suggesting that individual prevention by controlling the potential risk factors can be the best strategy to reduce the incidence of CRC [2,3]. Substantial progress, of course, has been made over decades in the treatment of various types of cancers, as well as CRC. However, tools of management are still based on surgery, chemotherapy, radiation, and use of anticancer drugs. Along with these treatments, searches for different modalities to treat cancer diseases have been stressed in clinics [4], and immunotherapy has recently come into the spotlight as a promising new anticancer strategy [5,6].
The immune system consists of multiple components, such as cells and molecules, that cross talk in response to surrounding stimuli, giving rise to immunity resulting from the integration of a variety of immune responses to sustain immune homeostasis [7,8]. Accordingly, breach of the immune system can cause serious problems in maintaining a physiological steady state. Indeed, numerous trials in both laboratories and clinics have been conducted in order to unveil the implications of the immune system for cancer progression, and many remarkable immune markers and phenomenon have been identified to explain cancer immunity [9].
As key players in the innate immune system, natural killer (NK) cells constitute one of three major lymphocyte subsets in all vertebrates. Since their first identification in a mouse study in 1975, NK cells have been ranked in the primary position as one of the most extensively studied subjects in immunology, and the focus has been on their ability of natural cytolytic activity against host-derived malignant cells in the absence of prior sensitization [10,11]. Because of the results from accumulated studies on the crucial functions of NK cells in tumor immunosurveillance, clinicians have started paying attention to the potential implications of NK cells for diagnosis, treatment and prognosis in tumor development and progression [12]. In vitro measurement of NK cell cytolytic activity by using cancer cell lines have been performed for a long time as one of the more reliable methods to assess cellular innate immunity in a variety of cancer diseases [13]. However, strictly speaking, the conventional method of NK-cell-based cytolytic activity measurement is likely to merely represent in vitro single-cell activity rather than the total cellular immunity generated from cellular interactions in the actual environment of the blood stream.
From this point of view, we devised a simple method in which a more substantial environment of cell-to-cell contact could be created using in vitro preparation of peripheral blood mononuclear cells (PBMCs). By considering fluctuations in the immune cells circulating in the blood, we assessed cytolytic activity of those immune cells in CRC patients and healthy donors to verify whether this method would be useful for evaluating cancer-related immunity.
Under the informed written consent according to the Institutional Review Board guidelines of Seoul Song Do Colorectal Hospital, peripheral whole blood was obtained randomly from CRC patients (n = 94) and healthy donors (n = 112) who were free from any kind of immune diseases. For patients, blood samples were obtained at one week before and after the laparoscopic surgery. Seven mililiters of heparinized blood collections were immediately processed by using Ficoll-hypaque density gradient centrifugation (GE healthcare, Uppsala, Sweden) at 1,500 g for 20 minutes at 28℃. Buffy coat layers including PBMCs were isolated and washed twice in phosphate-buffered saline (PBS). PBMCs were then centrifuged at 350 g for 5 minutes at 28℃ to completely remove PBS. The cell pellet was resuspended in 700 µL of complete RPMI 1640 media (Welgene, Daegu, Korea) with 5% (v/v) fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% (v/v) penicillin-streptomycin (Gibco). In parallel, 5 mL of ethylenediaminetetraacetic acid-treated blood collections were tested by using a Sysmex XE-2100 automated hematology analyzer (Sysmex, Kobe, Japan).
K562 cells, human leukemia cell line sensitive and specific to NK cells, were kindly gifted by Dr. Yeon-Sook Yun, Korea Institute of Radiological Medical Science, Seoul, Korea. The cells were maintained in complete media at 37℃ and 5% CO2 in a humidified incubator (Thermo, Marietta, OH, USA), and passaged at a 60-80% confluence to avoid cell overgrowth.
The cytolytic activity assay was done using CytoTox 96 Non-Radioactive assay kits (Promega, Madison, WI, USA). PBMC effector cells were prepared in 2 different ways. In the conventional method, 5 × 105 cells were counted and prepared for analysis, while in our "simple" method, 500 µL blood samples was analyzed in the absence of cell counting. 2 × 104 K562 target cells were mixed with different populations of the PBMC effector cells in triplicate and cocultured in 96-well round bottom plates for 2 hours at 37℃ in a tissue culture incubator. Subsequently, reaction plates were centrifuged at 250 g for 5 minutes, and 50 µL of culture media was transferred into a 96-well flat bottom plate to perform the lactate dehydrogenase-substrate enzymatic reaction. Optical density was measured from the reaction, and cytolytic activity was calculated by the following equation:
The remaining 400 µL of PBMCs from the cytolytic activity assay were used for staining with fluorescence conjugated monoclonal antibodies (mAbs) (BD Pharmingen, San Diego, CA, USA) to analyze NK cells. Fifty mililiters of PBMCs were labeled with Fluorescein isothiocyanate conjugated mouse antihuman CD3 mAbs and phycoerythrin conjugated mouse antihuman CD56 mAbs for 20 minutes at 4℃ in dark room and then washed in PBS with 4% (v/v) FBS by centrifugation at 350 g for 5 minutes at 4℃. The cell pellet was resuspended in 200 µL of PBS and assessed by flow cytometry (BD FACScalibur, San Jose, CA, USA). Data were analyzed by using CellQuest pro software (BD, San Jose, CA, USA).
Data were analyzed using the IBM SPSS ver. 18 (IBM Co., Armonk, NY, USA). First, a Kolmogorov-Smirnov test and a Shapiro-Wilks test were performed to check whether all variables conformed to normal distributions. A Student t-test for a Gaussian distribution and a Mann-Whitney U test for a non-Gaussian distribution were used to determine the significant differences between groups. A P-value less than 0.05 was considered to be significant.
Ninety-four patients (the patient group) with CRC and 112 healthy donors (the healthy group) were categorized by gender and age. The mean age was 51 years for the patients and 61 years for the healthy donors. The patient group consisted of 64 males and 30 females, and the healthy group consisted of 58 males and 54 females. The patient group was further classified by using the TNM staging system in order to investigate the association between cytolytic activity and cancer progression (Table 1).
Comparing our simple method to the conventional method of cytolytic activity measurement, we examined whether our simple method would be significantly useful for evaluating the activity of immune cells circulating in the blood of preoperative CRC patients. In the beginning, PBMC cytolytic activity against K562 cells was tested in both the patient (n = 18) and the healthy (n = 18) groups by using the conventional method. The mean value of the cytolytic activity for the preoperative patient group was significantly lower than that in the healthy group (6.45% ± 1.80% vs. 12.70% ± 7.70%, P < 0.001) (Fig. 1A). Simultaneously, PBMC cytolytic activity in the same cohorts was tested by using our simple method. The mean value of cytolytic activity for the preop patient group was also significantly lower than that in the healthy group (8.29%±2.12% vs. 17.81±9.57%, P < 0.001) (Fig. 1B).
Next, the cytolytic activity of immune cells circulating in the blood of CRC patients was explored to examine the influence of the surgical treatment. The cytolytic activity was significantly lower for both preop and postoperative patient groups than for the healthy group (8.82% ± 3.84% and 9.95% ± 4.94% vs. 17.02% ± 9.71 #x0025;, P < 0.001). However, no significance difference was observed between the preoperative and postoperative patient groups (8.82% ± 3.84% vs. 9.95% ± 4.94%, P = 0.326) (Fig. 2).
On account of the significant difference in the cytolytic activity between the patient and the healthy groups, we analyzed the phenotypic characteristics of CD3-CD56+NK cells, lymphocytes, and white blood cells to examine whether cellular changes could cause a decline in the cytolytic activity in the CRC patient group. NK cell numbers were not statistically different between the preop patient group and the healthy group (397 ± 253 cells/µL vs. 320 ± 133 cells/µL; P = 0.152); however, the percentage of NK cells was significantly increased for the preop patient group compared to that for the healthy group (19.97% ± 11.51% vs. 19.33% ± 9.83%, P = 0.041). Likewise, lymphocyte numbers were not different between the preop patient group and the healthy group. However, the percentage of lymphocytes was significantly decreased in the preop patient group compared to the healthy group (28.41% ± 8.31% vs. 36.4% ± 8.60%, P < 0.001). On the other hand, the numbers of white blood cell were not different between those two groups (7,346 ± 2,076 cells/µL vs. 7,231 ± 2,058 cells/µL, P = 0.734) (Table 2).
We examined the relationship between CRC and other clinical variables by using a multiple logistic regression analysis. We observed that CRC was significantly associated with age (odds ratio [OR], 0.905; confidence interval [CI], 0.867 to 0.944), cytolytic activity of PBMC (OR, 1.519; CI, 1.326 to 1.741), percentage of CD3-CD56+NK cells (OR, 0.942; CI, 0.891 to 0.997), and percentage of lymphocytes (OR, 1.095; CI, 1.033 to 1.160) (Table 3).
The cytolytic activity of immune cells circulating in the blood of CRC patients was analyzed according to the TNM classification. However, we could not find a statistically significant relationship between cytolytic activity and tumor stage (data not shown).
Conventional in vitro measurement of cellular cytolytic activity is a practical method for evaluating cellular activity and can be used to indirectly determine cellular immunity. However, this method is based on an artificial process in which effector cells and target cells are calculated by cell number optimized empirically in the laboratory, and then mixed in a certain ratio in reaction vessels. Although this is useful to some extent, the results from the conventional method do not accurately reflect true cellular immunity, because the immune cells and their target cells in the blood stream do not encounter each other in this manner.
In this context, we hypothesized that the measurement of cellular cytolytic activity by using a simple preparation of PBMCs and considering circadian fluctuations of the circulating blood immune cells might be an alternative method for evaluating the immunity of intact blood cells. Thus, we made an actual environment in which actual immune cells per se, in the blood, could encounter their target cells so as to assess the cytolytic activity of immune cells circulating in the blood. We analyzed the absolute numbers and the percentages of the lymphocytes and CD3-CD56+NK cells that play key roles in the reaction, since the number of PBMCs obtained from each subject varies according to the precise physiological conditions [14,15].
Our results showed that cytolytic activity is significantly reduced in the patient group compared to the healthy group. However, no significant differences in the absolute counts of lymphocytes and NK cells in the blood were identified between those two groups. This finding indicates that the activity of immune cells, including NK cell, in the blood is impaired in CRC patients, which is in agreement with the results in previous reports on cytolytic activity measurements performed by using the conventional method for various types of cancers [16-18].
Notably, we found phenotypic differences of CD3-CD56+NK cells and lymphocytes between CRC patients and healthy donors. This finding suggests that the defective cytolytic activity of circulating blood immune cells might be associated with phenotypic changes in the blood immune cells. In addition, multiple logistic regression analysis indicated that examination of cellular phenotypic changes combined with measurement of cytolytic activity can be used to functionally discriminate cancer patients from healthy individuals. In fact, many immune diseases are accompanied by changes in blood lymphocyte subsets during disease onset and progression. For instance, a decrease in CD4 helper T cells is vulnerable to human immunodeficiency virus infection [19], and the turnover of CD8 cytotoxic T cells and NK cells is related to several types of viral infections [20,21].
A limitation of this study was that we could not investigate the relation between cytolytic activity and cancer recurrence, because our study was performed for only one year, and this period was relatively short in acquiring data. However, we plan to address this issue and hope to have sufficient data to analyze in the near future. Another limitation lies in the fact that we did not asses the fitness of the patients with respect to surgical treatment that might influence immune status, because many studies have reported on surgical stress and immune suppression [22-24].
In conclusion, we demonstrate in this study that circulating blood immune cells of CRC patients are defective in function and phenotype, and that our simple method of cytolytic activity measurement, in combination with an analysis of the cellular phenotypic change, is useful for evaluating cellular immunity in CRC patients. Therefore, we suggest that a cellular functional assay should be run parallel to phenotypic assay in order to better understand cellular immunity. This report is our first step towards understanding cancer immunity, and a further large-scaled study is currently underway to clarify the role of the immune system in cancer.
Figures and Tables
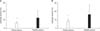 | Fig. 1Peripheral blood mononuclear cell (PBMC) cytolytic activity against K562 cells for two different methods in preoperative patients with colorectal cancer (n = 18) and healthy donors (n = 18). (A) 5 × 105 PBMCs were cocultured with 2 × 104 K562 cells in a ratio of 25:1 for 2 hours. (B) An unfixed number of PBMC isolated from 500 µL of blood was cocultured with 2 × 104 K562 cells for 2 hours. Values are expressed as mean ± standard deviation. *Designates that P-value against the healthy group is less than 0.001 (Student t-test). |
 | Fig. 2Peripheral blood mononuclear cell (PBMC) cytolytic activity against K562 cells in patients with colorectal cancer (n = 94) and in healthy donors (n = 112). An unfixed number of PBMCs derived from 500 µL of blood was cocultured with 2 × 104 K562 cells for 2 hours. Values are presented as mean ± standard deviation. *Signifies that P-value against the healthy group is less than 0.001 (Student t-test). |
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
2. Wu AH, Paganini-Hill A, Ross RK, Henderson BE. Alcohol, physical activity and other risk factors for colorectal cancer: a prospective study. Br J Cancer. 1987; 55:687–694.
3. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010; 138:2029–2043.e10.
4. Cao Y, DePinho RA, Ernst M, Vousden K. Cancer research: past, present and future. Nat Rev Cancer. 2011; 11:749–754.
5. Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001; 411:380–384.
6. Smith CL, Dulphy N, Salio M, Cerundolo V. Immunotherapy of colorectal cancer. Br Med Bull. 2002; 64:181–200.
7. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006; 6:24–37.
8. Sirisinha S. Insight into the mechanisms regulating immune homeostasis in health and disease. Asian Pac J Allergy Immunol. 2011; 29:1–14.
9. Hourigan CS, Levitsky HI. Evaluation of current cancer immunotherapy: hemato-oncology. Cancer J. 2011; 17:309–324.
10. Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002; 2:850–861.
11. Waldhauer I, Steinle A. NK cells and cancer immu nosurveillance. Oncogene. 2008; 27:5932–5943.
12. Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011; 2011:676198.
13. Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982; 155:1823–1841.
14. Miyawaki T, Taga K, Nagaoki T, Seki H, Suzuki Y, Taniguchi N. Circadian changes of T lymphocyte subsets in human peripheral blood. Clin Exp Immunol. 1984; 55:618–622.
15. Ritchie AW, Oswald I, Micklem HS, Boyd JE, Elton RA, Jazwinska E, et al. Circadian variation of lymphocyte subpopulations: a study with monoclonal antibodies. Br Med J (Clin Res Ed). 1983; 286:1773–1775.
16. Lin CC, Kuo YC, Huang WC, Lin CY. Natural killer cell activity in lung cancer patients. Chest. 1987; 92:1022–1024.
17. Piroozmand A, Hassan ZM. Evaluation of natural killer cell activity in pre and post treated breast cancer patients. J Cancer Res Ther. 2010; 6:478–481.
18. Tartter PI, Steinberg B, Barron DM, Martinelli G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg. 1987; 122:1264–1268.
19. Barnett D, Walker B, Landay A, Denny TN. CD4 immunophenotyping in HIV infection. Nat Rev Microbiol. 2008; 6:11 Suppl. S7–S15.
20. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006; 439:682–687.
21. Biron CA, Turgiss LR, Welsh RM. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983; 131:1539–1545.
22. Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, et al. Suppression of cellular immunity by surgical stress. Surgery. 2000; 127:329–336.
23. Espi A, Arenas J, Garcia-Granero E, Marti E, Lledo S. Relationship of curative surgery on natural killer cell activity in colorectal cancer. Dis Colon Rectum. 1996; 39:429–434.
24. Pollock RE, Lotzova E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991; 126:338–342.




 ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download