Abstract
Purpose
In this study, we investigated the therapeutic potential of regulated negative pressure vacuum-assisted wound therapy for inguinal lymphatic complications in critically ill, liver transplant recipients.
Methods
The great saphenous vein was harvested for hepatic vein reconstruction during liver transplantation in 599 living-donor liver transplant recipients. Fourteen of the recipients (2.3%) developed postoperative inguinal lymphatic complications and were treated with negative pressure wound therapy, and they were included in this study.
Results
The average total duration of negative pressure wound therapy was 23 days (range, 11 to 42 days). Complete resolution of the lymphatic complications and wound healing were achieved in all 14 patients, 5 of whom were treated in hospital and 9 as outpatients. There was no clinically detectable infection, bleeding or recurrence after an average follow-up of 27 months (range, 7 to 36 months).
Lymphatic complications, such as lymphorrhea, lymphocutaneous fistula, and lymphocele, are uncommon after saphenous vein harvest in the inguinal area, but their treatment represents a serious challenge especially in critically ill patients [1-3]. Current treatment modalities for lymphatic complications are time consuming and moderately successful. Recent data suggest that creating a subatmospheric pressure by vacuum-assisted closure therapy supports the wound healing process [4-6].
In our institution, we used the autogenous great saphenous vein for hepatic vein reconstruction during living-donor liver transplantation [7,8]. There is an increased risk of infection, bleeding and delayed wound healing in liver transplant recipients with inguinal wounds, because of immunosuppresion, immediate postoperative deterioration of liver functions and poor nutritional status. The purpose of this study was to investigate the therapeutic potential of regulated negative pressure vacuum-assisted wound therapy for inguinal lymphatic complications in living-donor liver transplant recipients in whom the great saphenous vein was harvested for hepatic vein reconstruction during liver transplantation.
Between January 2010 and December 2011, the great saphenous vein was harvested for hepatic vein reconstruction during liver transplantation in 599 living-donor liver transplant recipients, and the clinical details are summarized in Table 1. Of these, 14 patients (2.3%) developed postoperative lymphatic complications, even though lymphatic drainage was performed through a Jackson-Pratt Drain in the inguinal wound. These 14 patients were treated with negative pressure wound therapy and are the subjects of this study. The following demographic and medical background variables were recorded for all patients: age, gender, body mass index (BMI), presence of risk factors, indications of liver transplantation, type of lymphatic complications, time from surgery to application of negative pressure wound therapy, total duration of negative pressure wound therapy, and follow-up information. This study is a prospective exploratory study with approval from the Institutional Review Board. All information pertaining to the subjects were used in compliance with Korean legislation and all the participants gave written informed consent. All data analysis was performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA).
Conventional immunosuppressive therapy for adult liver transplant recipients at Asan Medical Center consisted of interleukin-2 receptor inhibitor (basiliximab) on days 0 and 4; an intraoperative steroid bolus (5-10 mg/kg), intravenous or oral calcineurin inhibitor (CNI) and corticosteroid recycling since day 1; adjunctive mycophenolate mofetil (MMF) for patients showing CNI-associated side effects or for immunosuppressive augmentation for suspected mild acute cellular rejection. For oral CNI, tacrolimus was usually preferred, but tacrolimus and cyclosporine were occasionally exchanged for control of CNI-associated side effects. The target tacrolimus concentrations were around 15 ng/mL for the first two weeks; 12 ng/mL for months 1-3; 8-10 ng/mL within one year; 5-8 ng/mL for years 1-2; 5 ng/mL for years 3-5; and below 5 ng/mL after five years. The two main reasons for combined tacrolimus and MMF therapy were to reduce tacrolimus concentration (CNI sparing) to relieve nephrotoxicity or other side effects and to augment immunosuppressive activity (MMF add-on) against unstable liver enzyme levels. MMF monotherapy was avoided during the first three months except for very unusual circumstances such as serious side effects from CNIs; for intractable nephrotoxicity of CNIs, combined immunosuppressive therapy and hemodiafiltration were performed. After the first three months, combination therapy with minimal CNI was attempted for patients showing intractable intolerance to CNIs [9].
The diagnosis of lymphatic complications was made clinically based on continued drainage of large amounts of clear fluid (>150 mL/day, for >72 hours) from the inguinal incision, continued drainage through the disrupted inguinal wound or the presence of a cystic collection of lymphatic fluid in the soft tissue of the healing wound: these complications were categorized as lymphorrhea (continued clear fluid drainage through the Jackson-Pratt Drain without wound disruption), lymphocutaneous fistula (continued clear fluid drainage through the disrupted wound) and lymphocele (a cystic collection of lymphatic fluid in the healing wound). The initial therapeutic approach for lymphatic complications was conservative treatment: frequent heavy dressings with bed rest for lymphorrhea and lymphocutaneous fistula, and percutaneous aspiration to relieve patient symptoms for lymphocele. In this study, indications for negative pressure wound therapy were that 1) lymphatic drainage was not decreasing 72 hours after conservative treatment; 2) continued drainage increased the risk of inguinal wound infection; and 3) the patient had symptomatic recurrence after multiple aspirations.
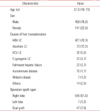
For negative pressure wound therapy, we used a regular black sponge polyurethane foam dressing with a continuous negative pressure of 125 mmHg (CuraVAC, BioAlpha Inc., Seongnam, Korea) (Fig. 1). The dressings were changed twice a week in the hospital. Once adequate control of drainage was obtained and transplanted liver functions were normal, the patient were discharged home with a portable suction unit. They measured the amount of drainage daily and attended the outpatient clinic for dressing change every week until the wounds were completely healed. The decision to stop the negative pressure wound therapy was based on minimal drainage (<10 mL/day) from the wound for 2-3 days and good wound healing in terms of granulation tissue formation. After completion of the negative pressure wound therapy, delayed primary closure was performed and none of the wounds required skin grafting.
Fourteen patients who developed inguinal lymphatic complications following living-donor liver transplantation were treated with negative pressure wound therapy: 11 lymphorrhea, 2 lymphocutaneous fistula and 1 lymphocele. The male-to-female ratio was 9:5, and the mean age and BMI of the patients were 51 years (range, 33 to 64 years) and 26.11 kg/m2 (range, 18.23 to 31.87 kg/m2), respectively. Two patients had comorbid diabetes mellitus; 2 had comorbid hypertension; and 6 were current smokers. Indications for living-donor liver transplantation in these patients were hepatitis B cirrhosis with hepatocellular carcinoma (n = 8), hepatitis B cirrhosis without hepatocellular carcinoma (n = 3), autoimmune disease (n = 1), and Wilson's disease (n = 2). The transplant recipients were treated with conventional immunosuppressive therapy after transplantation.
Complete wound healing was achieved in all 14 patients, 5 of whom were treated in hospital and 9 as outpatients. The findings relating to time from transplantation to complete wound healing for all the cases and across type of lymphatic complications are provided in Table 2. The median duration between transplantation and application of negative pressure wound therapy was 20 days (range, 3 to 68 days), and the average total duration of negative pressure wound therapy was 23 days (range, 11 to 42 days). In the 9 patients discharged with portable suction units, the average total duration of negative pressure wound therapy in the outpatient clinic was 13 days (range, 7 to 21 days). The success rate in achieving complete resolution of lymphatic complications with negative pressure wound therapy was 100%, as no clinically detectable infection, bleeding or recurrence has been reported after an average follow-up of 27 months (range, 7 to 36 months).
Inguinal lymphatic complications have been reported after lymph node biopsy, arterial reconstruction, vascular cannulation, saphenous vein harvest, and other procedures, and are usually attributed to damage during dissection in the region of the femoral neurovascular bundle without detailed ligation of small lymphatics [1-4,10]. Despite all efforts to prevent lymphatic complications, the overall reported incidence ranges from 1.2% to 5.1% [1,2,11,12]. Patients with lymphatic complications have increased length of hospital stay, ranging from 22 to 36 days, and significantly increased risk of wound infection, reportedly up to 18% [11,12]. Therefore, uncontrolled lymphatic drainage can be the source of significant morbidity and mortality for critically ill patients.
Although several therapeutic options have been described for the treatment of lymphatic complications, these options have variable degrees of success and there is no consensus in the literature regarding the preferred treatment [1]. Nonoperative recommendations have included bed rest, prophylactic antibiotics, and pressure dressings. This management results in extended hospital stays, increased cost, patient immobility, and risk of underlying wound infection [2]. Operative modalities, involving early identification by surgical exploration and lymphatic ligation, have been advocated by some authors, but this exposes the patient to extensive dissection and another operation, which may increase the cost and duration of hospital stay [1,2,13,14]. Especially in liver transplant recipients with immunosuppresion, postoperative deterioration of liver function and poor nutritional status, the greatly increased risk of infection and bleeding, and delayed wound healing, also become significant issues when major surgical reinterventions are contemplated in these poor-risk patients.
Since its introduction in 1995 as a wound treatment modality, negative pressure wound therapy, creating a subatmospheric pressure with a vacuum-assisted closure device, has proved to be one of the most effective methods of managing all types of wounds [1,2,6,15-19]. This dressing technique consists of putting an open cell foam dressing into the wound cavity, connecting it to a vacuum pump with a tube and covering it with an adhesive drape [20]. Negative pressure wound therapy exposes the wound bed to a negative pressure able to remove fluid from the extravascular space along with inflammatory mediators, which are detrimental to wound healing, improves circulation and promotes wound healing and granulation tissue formation as well as reducing bacterial load [15-22]. Currently, it has been used for the traetment of pressure sores, stasis ulcers, chronic wounds such as dibetic foot ulcers, post-traumatic and postoperative wounds, infected wounds such as necrotizing fasciitis or sternal wounds, soft tissure injuries, bone-exposed injuries and abdominal open wounds, as well as for securing skin grafts [20]. Although many approaches for the treatment of lymphatic complications have been described, no one mode has clearly emerged as the best solution. Some authors have reported that negative pressure wound therapy is superior to conventional nonoperative or operative treatment modalities in the management of inguinal lymphatic complications [1-4]. In our experience, negative pressure wound therapy results in rapid resolution of lymphatic complications, is amenable to outpatient management, and decreases the time to closure compared with existing treatment options and without the morbidity of an operative procedure.
In our study, we achieved a success rate of 100% in obtaining complete resolution of inguinal lymphatic complications with negative pressure wound therapy in 14 liver transplant recipients. This method of management offers early control of fluid drainage, rapid control of the wound, earlier closure, and the potential for reduced length of stay, and eventually decreases the danger of significant morbidity and mortality for critically ill patients. Moreover, in appropriately motivated individuals, patient acceptance and convenience can be enhanced by outpatient management with small portable home devices and the ability to return to work [1]. Although we are not suggesting that negative pressure wound therapy should be the initial treatment option for lymphatic complications, our results suggest that it is a safe and effective alternative treatment option for failed, uncontrollable lymphatic complications, especially in critically ill patients.
In conclusion, negative pressure wound therapy is a safe and effective, readily-available treatment option that is less invasive than exploration and ligation of leaking lymphatics and provides easy control of drainage, and rapid wound closure.
Figures and Tables
 | Fig. 1Application of negative pressure wound therapy, consisting of putting a conventional black sponge polyurethane foam dressing into the inguinal wound cavity, connecting it to a vacuum pump with a tube and covering it with an adhesive drape. |
References
1. Hamed O, Muck PE, Smith JM, Krallman K, Griffith NM. Use of vacuum-assisted closure (VAC) therapy in treating lymphatic complications after vascular procedures: new approach for lymphoceles. J Vasc Surg. 2008; 48:1520–1523.e4.
2. Abai B, Zickler RW, Pappas PJ, Lal BK, Padberg FT Jr. Lymphorrhea responds to negative pressure wound therapy. J Vasc Surg. 2007; 45:610–613.
3. Dosluoglu HH, Loghmanee C, Lall P, Cherr GS, Harris LM, Dryjski ML. Management of early (<30 day) vascular groin infections using vacuum-assisted closure alone without muscle flap coverage in a consecutive patient series. J Vasc Surg. 2010; 51:1160–1166.
4. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg. 2006; 44:1029–1037.
5. Labanaris AP, Polykandriotis E, Horch RE. The effect of vacuum-assisted closure on lymph vessels in chronic wounds. J Plast Reconstr Aesthet Surg. 2009; 62:1068–1075.
6. Shweiki E, Gallagher KE. Negative pressure wound therapy in acute, contaminated wounds: documenting its safety and efficacy to support current global practice. Int Wound J. 2013; 10:13–43.
7. Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, et al. Standardization of modified right lobe grafts to minimize vascular outflow complications for adult living donor liver transplantation. Transplant Proc. 2012; 44:457–459.
8. Hwang S, Lee SG, Ahn CS, Moon DB, Kim KH, Sung KB, et al. Morphometric and simulation analyses of right hepatic vein reconstruction in adult living donor liver transplantation using right lobe grafts. Liver Transpl. 2010; 16:639–648.
9. Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A clinical assessment of mycophenolate drug monitoring after liver transplantation. Clin Transplant. 2010; 24:E35–E42.
10. Slappy AL, Hakaim AG, Oldenburg WA, Paz-Fumagalli R, McKinney JM. Femoral incision morbidity following endovascular aortic aneurysm repair. Vasc Endovascular Surg. 2003; 37:105–109.
11. al-Salman MM, Rabee H, Shibli S. Groin lymphorrhea postoperative nuisance. Int Surg. 1997; 82:60–62.
12. Tyndall SH, Shepard AD, Wilczewski JM, Reddy DJ, Elliott JP Jr, Ernst CB. Groin lymphatic complications after arterial reconstruction. J Vasc Surg. 1994; 19:858–863.
13. Shermak MA, Yee K, Wong L, Jones CE, Wong J. Surgical management of groin lymphatic complications after arterial bypass surgery. Plast Reconstr Surg. 2005; 115:1954–1962.
14. Stadelmann WK, Tobin GR. Successful treatment of 19 consecutive groin lymphoceles with the assistance of intraoperative lymphatic mapping. Plast Reconstr Surg. 2002; 109:1274–1280.
15. Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997; 38:563–576.
16. Salman AE, Yetisir F, Aksoy M, Tokac M, Yildirim MB, Kilic M. Use of dynamic wound closure system in conjunction with vacuum-assisted closure therapy in delayed closure of open abdomen. Hernia. 2012; 10. 30. [Epub]. http://dx.doi.org/10.1007/s10029-012-1008-0.
17. Grauhan O, Navasardyan A, Hofmann M, Muller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg. 2013; 145:1387–1392.
18. Li J, Topaz M, Tan H, Li Y, Li W, Xun W, et al. Treatment of infected soft tissue blast injury in swine by regulated negative pressure wound therapy. Ann Surg. 2013; 257:335–344.
19. Ousey KJ, Milne J, Cook L, Stephenson J, Gillibrand W. A pilot study exploring quality of life experienced by patients undergoing negative pressure wound therapy as part of their wound care treatment compared to patients receiving standard wound care. Int Wound J. 2012; 10. 24. [Epub]. http://dx.doi.org/10.1111/j.1742-481X.2012.01098.x.
20. Chiummariello S, Guarro G, Pica A, Alfano C. Evaluation of negative pressure vacuum-assisted system in acute and chronic wounds closure: our experience. G Chir. 2012; 33:358–362.
21. Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997; 38:553–562.
22. Wackenfors A, Sjoren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen. 2004; 12:600–606.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download