Abstract
Purpose
To compare and assess the efficacy, safety and utility of hand-assisted laparoscopic surgery (HALS) with open surgery (OS) in total colectomy with ileorectal for colonic inertia.
Methods
From January 2001 to February 2012, 56 patients diagnosed with colonic inertia who failed to respond to medical treatments underwent hand-assisted laparoscopic total colectomy with ileorectal anastomosis. Another 68 patients underwent laparotomy. Main parameters such as clinical manifestations, conversion to open procedure, operative time, incision length, pain score, intraoperative blood loss, time to first flatus and hospitalization, early postoperative complications and hospitalization cost were retrospectively analyzed. Postoperative defecating frequencies were followed up in both groups.
Results
All patients received successful operation, no surgical mortality happened and none of the patients required conversion to an exploratory laparotomy in HALS group. The clinical features, the estimated blood loss, incision length, pain score, first passing flatus time, and postoperative hospitalization time were superior in HALS group (P < 0.05). The early postoperative complications and frequency of defecation were similar. However, the mean operative time was longer and hospitalization cost was higher in HALS group than those in OS group (P < 0.05).
Colonic inertia is a common cause of chronic functional constipation. It mainly brings about slow colonic transit, leading to symptoms such as severe abdominal pain, abdominal fullness, and nausea. These patients usually have a long history of laxative abuse and turn dependent on these drugs. However, a patient with colonic inertia who fails to respond to medical therapy may finally need surgery. There have been various colorectal operations developed for colonic inertia. Nonetheless, several surgeons have demonstrated that the better surgical treatment was total colectomy with an ileorectal anastomosis [1,2]. In recent years, minimally invasive approaches, such as laparoscopic surgery, are widely accepted in general surgery and gradually becoming a preferred routine technique in colorectal surgery as well. The immediate benefits are obvious, but the disadvantages include loss of direct tactile sensation, hand-eye coordination during laparoscopic surgery and longer time for training, especially in extensive colorectal procedures such as total colectomy [3]. In 1994, Leahy et al. [4] designed the hand-assisted devices for laparoscopic colon surgery, This hybrid operation allows the surgeon to introduce a hand into the abdominal cavity through a special hand port while maintaining the pneumoperitoneum [5]. Several studies have demonstrated that hand-assisted lapa roscopic surgery (HALS) could be a more preferable procedure than standard laparoscopic surgery or open total colectomy [5,6]. However, few studies have ever focused on HALS with total colectomy for the treatment of colonic inertia. Consequently, the purpose of this study is to evaluate the safety and feasibility of hand-assisted laparoscopic total colectomy compared with traditional laparotomy for colonic inertia.
We carried out a retrospective analysis of 124 consecutive patients who were diagnosed with colonic inertia and failed to respond to medical treatments from January 2001 to February 2012 in Department of Colorectal Surgery, The First Affiliated Hospital, Zhejiang University. In all cases, careful clinical evaluations (containing digital rectal examination and psychological consult) were performed and the disease was established with the diagnostic workup including: colonoscopy, defecography, colonic transit test, manometry, and balloon expulsion. Prudently, we defined a positive colonic transit test as any patient who had more than 20% of radiopaque markers still present in the colorectum after 120 hours. Anal manometry, defecography, and balloon expulsion test were conducted to assure no outlet obstructed defecation. Colonoscopy was done to ensure that no mechanical obstruction such as tumor accounted for the constipation or other colorectal pathological changes. Eligible patients were those in complete accord with the positive colonic transit test, but the colonoscopy, defecography, manometry, and balloon expulsion were shown to be normal. Fifty-six patients underwent a HALS total colectomy with ileorectal anastomosis. Another 68 patients underwent open surgery (OS).
The two groups' patients received similar perioperative management during the whole hospitalization. Preoperatively, all patients underwent mechanical bowel preparation; injected antibiotics thirty minutes before operation. Postoperatively, pain management with patient controlled analgesia was performed. All patients were treated with antibiotics and total parenteral nutrition (TPN), stopped TPN and recovered liquid diet when first passage of flatus happened.
The operation was performed with the patient in the lithotomy
position, the surgeon stood between the two legs of the patient. Initially, A 10-mm trocar was passed into the abdomen through a small incision made just above the umbilicus. A 10-mm laparoscope was inserted through the supraumbilical trocar, and a diagnostic laparoscopy was performed after a satisfactory pneumoperitoneum was established. Then a LapDisc (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA), the hand-assisted device, could be placed through a 6- to 7-cm transverse incision in the lower abdomen (about 3 cm above pubic bone), the assisted hand was sequentially put into the abdominal cavity through the LapDisc. Moreover, another one or two 10-mm trocar was inserted in the left or right lower quadrant for the insertion of the ultrasonic scalpel (Fig. 1). The colon marginal or the terminal blood vessels were ligated by ultrasonic scalpel to liberate the mesocolon in the order of sigmoid colon, descending colon, transverse colon, ascending colon, and cecum. Then, the mobilized total colon was brought out through the LapDisc and the ileorectal end-to-end anastomosis was performed by the Valtrac anastomosis ring (Tyco, Princeton, NJ, USA) under direct vision. Finally, we closed the mesenteric slit pore and the wound with a closed drain in the pelvis. In order to avoid mutilation of rectus abdominis and to get a better cosmetic appearance, since 2007, the position of LapDisc has been changed to surround the umbilicus. Then, four 10-mm trocars were inserted 3-4 cm below the xiphoid, in the left and right midabdomen (at the level of umbilicus), and in the suprapubic region, respectively, for laparoscope and ultrasonic scalpel (Fig. 2). We found that the modified incision became smaller and imperceptible after several months because of constriction of the umbilicus.
The operation was performed with the patient in the lithotomy position, as well. The abdomen was entered via a standard midline laparotomy with an 18- to 25-cm incision and the entire procedure including mobilization, resection, anastomosis and draining was similar with HALS except that it was performed under direct vision.
Two groups' data including clinical manifestations, conversion to open procedure, operative time, intraoperative blood loss, incision length, pain score, time to first flatus and hospitalization, early postoperative complications, and hospitalization cost were retrospectively analyzed by SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) using the t-tests (measurement data) or chi-square test (enumeration data) where appropriate. A P-value < 0.05 was considered to be statistically significant. Postoperative defecating frequencies of the patients were observed continuously for at least for 12 months in both groups.
All 124 patients received successful total colectomy with ileorectal anastomosis (56 HALS vs. 68 OS), no surgical mortality or severe complication happened during operation in both groups and no conversion to an exploratory laparotomy was required in HALS group. The clinical features such as age, gender, body mass index, course of disease, and complicated diseases were well matched (Table 1). Surgical results are presented in Table 2, showing that the estimated blood loss, incision length, pain score, first passing of flatus time, and postoperative hospitalization time were better in HALS group (P < 0.05). The early postoperative complications were similar. But the mean operative time was longer and hospitalizing cost was higher in HALS group than those in OS group (P < 0.05). The constipation symptoms were significantly relieved, and the defecation frequency was 4-6 times per day in both groups after two weeks of surgery. All of the patients in HALS group were satisfied with the appearance of the abdominal scar. Patients were followed up for more than 12 months, and the defecating frequencies were found to be similar in both groups at the 12th month. Two patients in OS developed adhesive intestinal obstruction within 3 months after surgery, who then received appropriate medical treatments. Two patients in HALS and 4 in OS group had constipation recurrence and needed some laxatives again after several months.
Pathology examination was conducted on all specimens. Intestinal mesenteric ganglion cells decreased in 112 cases out of the total 124 cases, with absence in the other 12 cases, 74 cases reported complicated with melanosis mucosa.
The term colonic inertia is characterized by prolonged delay in the transit of stool through the colon without any other underlying causes such as systemic disorder, mechanical ileus or pelvic floor dysfunction. The most common clinical features include refractory constipation, abdominal pain, abdominal distension, and nausea. Necessary physiological examinations include colonic transit test, colonoscopy, anal manometry, defecography, and balloon expulsion [7,8]. In this study, all eligible patients showed positive results in colonic transit test, but the colonoscopy, defecography, manometry, and balloon expulsion showed to be normal. Pathologically, this disease may be caused by enteric neuropathy resulting from abnormality of the enteric nervous system. Several studies revealed that this abnormality was considered to be related to a decrease in the interstitial cell of Cajal, which was recognized as an intestinal pacemaker and mainly situated in the submucosal and myenteric plexus of the bowel wall [2,7,9]. On the contrary, Toman et al. [10] demonstrated that the decreased numbers of interstitial cells of Cajal did not significantly contribute to colonic inertia. Accordingly, the nosetiology of the disease still remained uncertain. In our study, intestinal mesenteric ganglion cells decreased in 112 cases of 124 cases, with absence in the other 12 cases, which may support the former viewpoint. Many patients with colonic inertia have a long history of laxative abuse and become dependent on these drugs. Unfortunately, surgery usually is the final choice for the patient with severe, unremitting constipation who fails to respond to medical therapy. There have been various colorectal operations developed for colonic inertia. However, several surgeons had demonstrated that the better surgical treatment for colonic inertia was a total colectomy with an ileorectal anastomosis [1].
Generally speaking, there are three types of procedures that could be selected for total colectomy with an ileorectal anastomosis: OS, laparoscopic surgery, and HALS. As a benign colon disease, the patients are usually young, active, and highly motivated individuals who desire a cosmetically appealing and functional result [11]. Patients who require this operation for colonic inertia could be good candidates for minimally invasive surgery (MIS).
Laparoscopic technique has been currently adopted diffusely as a predominant MIS. Laparoscopic colorectal surgery was introduced in 1991 [12], which is usually associated with time-consuming, technically demanding, high conversion rate and has a long, steep learning curve [3,13,14], since surgeons need to handle a long mobile colon, and operate on multiple abdominal quadrants. Consequently, a new surgical procedure called HALS was introduced in the mid 1990s as a useful alternative to pure laparoscopic procedures [15]. This hybrid operation allows the surgeon to introduce the nondominant hand into the abdominal cavity through a special hand port while maintaining the pneumoperitoneum [4]. The most suitable operations for HALS are those that require extraction of a specimen and therefore necessitate an incision anyway [16], so it is a natural fit for HALS total colectomy. With the special hand port device, surgeons regain tactile feedback, can complete blunt dissection, retraction, control of bleeding, and organ removal simply [17]. Especially, if the surgeon is inexperienced in laparoscopic operations, HALS in some difficult parts of the operation may be useful [18].
Compared with OS, the majority of existing reports, especially in three RCTs [19-21], HALS is associated with less blood loss, less pain, higher cosmesis scores, faster postoperative recovery, shorter length of hospital stay and incision than OS but longer operative time and higher cost. Moreover, there is no difference in the complication occurrence, morbidity and functional outcome. Hsiao et al. [7] carried out HALS total colectomy on 44 patients with colonic inertia and compared his results with those of open procedure, which were demonstrated by Webster and Dayton [1] for the same disease. As a result, the former provided better clinical utility: Respectively, hospital stay (7.6 days vs. 10 days), prolonged ileus (11.4% vs. 24%), small bowel obstruction (4.5% vs. 4%), and mean bowel frequency (2.3 times per day vs. 3 times per day). In our study, less blood loss, smaller incision, lower pain score, faster first passing flatus, and shorter postoperative hospitalization time were observed in HALS group compared with OS group, which demonstrated the superiority of HALS in minimal invasiveness and fast recovery. The security was confirmed due to the similar early postoperative complication rates. We believe the operative time will become shorter as surgeons become more skilled. Although the hospitalizing cost was higher in HALS group, it was worthwhile considering the benefits.
In summary, HALS total colectomy with ileorectal anastomosis for colonic inertia is feasible and safe. It significantly reduces invasiveness compared with laparotomy while maintaining blood loss and postoperative complications. It combines the advantages of both laparoscopic (minimally invasive) and conventional OS [14]. However, it is a retrospective study and more randomized controlled trials are needed to further define the potential benefits of HALS over conventional OS for colonic inertia.
Figures and Tables
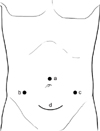 | Fig. 1Trocar and device placement. a, supraumbilical port for laparoscope; b and c, accessory port for harmonic scalpel; d, suprapubic transverse incision for LapDisc system. |
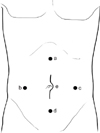 | Fig. 2Trocar and device placement. a-d, accessory port for laparoscope and harmonic scalpel; e, incision for LapDisc system. |
References
1. Webster C, Dayton M. Results after colectomy for colonic inertia: a sixteen-year experience. Am J Surg. 2001; 182:639–644.
2. Sohn G, Yu CS, Kim CW, Kwak JY, Jang TY, Kim KH, et al. Surgical outcomes after total colectomy with ileorectal anastomosis in patients with medically intractable slow transit constipation. J Korean Soc Coloproctol. 2011; 27:180–187.
3. Ozturk E, Kiran RP, Remzi F, Geisler D, Fazio V. Hand-assisted laparoscopic surgery may be a useful tool for surgeons early in the learning curve performing total abdominal colectomy. Colorectal Dis. 2010; 12:199–205.
4. Leahy PF, Bannenberg JJ, Meijer DW. Laparoscopic colon surgery: a difficult operation made easy. Surg Endosc. 1994; 8:992.
5. Nakajima K, Lee SW, Cocilovo C, Foglia C, Sonoda T, Milsom JW. Laparoscopic total colectomy: hand-assisted vs standard technique. Surg Endosc. 2004; 18:582–586.
6. Nakajima K, Nezu R, Hirota M, Nishida T. The role of hand-assisted laparoscopic surgery in subtotal and total colectomy for Crohn's colitis. Surg Endosc. 2010; 24:2713–2717.
7. Hsiao KC, Jao SW, Wu CC, Lee TY, Lai HJ, Kang JC. Hand-assisted laparoscopic total colectomy for slow transit constipation. Int J Colorectal Dis. 2008; 23:419–424.
8. Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am. 2003; 32:659–683.
9. Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006; 12:4609–4613.
10. Toman J, Turina M, Ray M, Petras RE, Stromberg AJ, Galandiuk S. Slow transit colon constipation is not related to the number of interstitial cells of Cajal. Int J Colorectal Dis. 2006; 21:527–532.
11. Ky AJ, Sonoda T, Milsom JW. One-stage laparoscopic restorative proctocolectomy: an alternative to the conventional approach? Dis Colon Rectum. 2002; 45:207–210.
12. Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc. 1991; 1:144–150.
13. Reichenbach DJ, Tackett AD, Harris J, Camacho D, Graviss EA, Dewan B, et al. Laparoscopic colon resection early in the learning curve: what is the appropriate setting? Ann Surg. 2006; 243:730–735.
14. Meshikhes AW. Controversy of hand-assisted laparoscopic colorectal surgery. World J Gastroenterol. 2010; 16:5662–5668.
15. Ou H. Laparoscopic-assisted mini laparatomy with colectomy. Dis Colon Rectum. 1995; 38:324–326.
16. Litwin DE, Darzi A, Jakimowicz J, Kelly JJ, Arvidsson D, Hansen P, et al. Hand-assisted laparoscopic surgery (HALS) with the HandPort system: initial experience with 68 patients. Ann Surg. 2000; 231:715–723.
17. Liu FL, Lin JJ, Ye F, Teng LS. Hand-assisted laparoscopic surgery versus the open approach in curative resection of rectal cancer. J Int Med Res. 2010; 38:916–922.
18. Kim HJ, Choi GS, Park JS, Park SY, Choi WH, Ryuk JP. Early postoperative and long-term oncological outcomes of laparoscopic treatment for patients with familial adenomatous polyposis. J Korean Surg Soc. 2012; 83:288–297.
19. Chung CC, Ng DC, Tsang WW, Tang WL, Yau KK, Cheung HY, et al. Hand-assisted laparoscopic versus open right colectomy: a randomized controlled trial. Ann Surg. 2007; 246:728–733.
20. Maartense S, Dunker MS, Slors JF, Cuesta MA, Gouma DJ, van Deventer SJ, et al. Hand-assisted laparoscopic versus open restorative proctocolectomy with ileal pouch anal anastomosis: a randomized trial. Ann Surg. 2004; 240:984–991.
21. Kang JC, Chung MH, Chao PC, Yeh CC, Hsiao CW, Lee TY, et al. Hand-assisted laparoscopic colectomy vs open colectomy: a prospective randomized study. Surg Endosc. 2004; 18:577–581.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download