Abstract
Purpose
The cervical lymph nodes are the most common sites of locoregional recurrence in patients with papillary thyroid carcinoma (PTC). Accurate tumor localization is important for the successful removal of impalpable recurrences in the cervical lymph nodes. We evaluated the benefits of ultrasound-guided localization (UGL) performed by a single surgeon on site.
Methods
Of 53 PTC patients who underwent reoperation for impalpable nodal recurrences, 32 (group 1) were assessed only using preoperative imaging, while 21 (group 2) were additionally evaluated by on-site UGL performed by the operating surgeon. Postoperative outcomes were compared between the two groups.
Results
Operation times were significantly shorter (P < 0.001) and the mean size of the resected lymph nodes were smaller (P = 0.013) for group 2 patients. More lymph nodes were identified and resected in group 1 (3.56 vs. 3.19), but the rate of positive lymph nodes was significantly higher in group 2 (P < 0.001). There were no differences between the two groups in terms of resection success rate, complication rate, and postoperative hospital stay. During a mean follow-up period of 27.6 months, 52 patients (98.1%) showed no evidence of recurrence on routine ultrasound, and serum thyroglobulin concentrations remained < 1 ng/mL in 49 patients (92.5%).
Although patients with papillary thyroid carcinoma (PTC) have excellent prognosis, between 5% and 40% have persistent or recurrent disease even after curative surgery [1,2]. Assays of serum thyroglobulin (Tg) concentrations combined with high-resolution ultrasound are widely used for postoperative follow-up, enabling early detection of recurrence and more prompt management [3-8].
Most locoregional recurrences develop in the cervical lymph nodes, and the majority can be controlled by surgical removal if there is no extensive invasion or distant metastasis [1-3,9-13]. Difficulties may be encountered, however, during re-exploration of the neck. For example, extensive scarring and fibrosis resulting from previous surgery can distort normal anatomic landmarks, prolonging operation time and increasing the rate of operative morbidities [13,14]. These problems may occur more frequently when the recurrent lesions are impalpable. Thus, accurate localization of recurrent lesions may be an important factor for successful outcomes.
Several methods have been described for the localization of impalpable, recurrent lesions, including radioiodine-detected probe-guided surgery [15], intraoperative ultrasound [16], preoperative tattooing with charcoal [17], hook-needle positioning in the lesion [18], and preoperative skin marking under ultrasound guidance [19].
On-site ultrasound-guided localization (UGL) by surgeon has recently been introduced at our institution. We therefore investigated the benefits of on-site UGL for impalpable nodal recurrences in PTC patients.
From March 2007 to September 2009, a total of 53 PTC patients underwent surgery for the removal of impalpable nodal recurrences in the central compartment (level 6) of the neck at the Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine. All patients had previously undergone a total thyroidectomy and central compartment lymph node dissection. Ultrasound follow-up in all patients showed nodal recurrences in single focus, which was confirmed by fine needle aspiration cytology. Patients with lateral neck or mediastinal lymph node recurrences were excluded from the study because of the variety of surgical approaches and operative procedures. All patients also underwent a computed tomographic (CT) scan of the neck. Chest radiography, CT scans of the chest and abdomen, and/or positron emission tomography (PET) were selectively performed to assess distant metastases.
The medical records of all patients were retrospectively reviewed, and the study protocol was approved by the Institutional Review Board of the hospital.
The patients were divided into two groups. Patients in group 1 (n = 32) underwent surgery based on the findings of the preoperative ultrasound and neck CT scans. Surgeons predicted the location of recurrent lymph node by estimating the distance from nearby structures (structures like internal jugular vein, common carotid artery, sternal notch) that were shown in previously performed imaging studies. No marking or tattooing procedures were performed for group I patients. Patients in group 2 (n = 21) additionally underwent on-site UGL in the operating room. UGL was performed after general anesthesia and positioning for surgery (neck extended position). Under ultrasound guidance, about 0.1 mL of methylene blue dye was injected into the recurrent lesion. All UGLs were performed by a single surgeon using an ALOKA diagnostic ultrasound system (Prosound SSD-3500 plus, Tokyo, Japan) and a 12-MHz linear transducer (Fig. 1).
All operations were performed by an experienced thyroid surgeon who performs more than 800 cases of thyroid surgery annually as the main operator. Previous operation sites were used for surgical approach and no patient required extension of the previous incision.
Surgical success was defined as: 1) pathologic confirmation of recurrence in the resected lymph node; 2) disappearance of the lesion on postoperative ultrasound, which was performed within a month after surgery. The ultrasound of each patient was performed by the same radiologist who had performed the fine needle aspiration. We compared the resection success rate, operation time, mean size of the resected lymph nodes, complication rate, and length of postoperative hospital stay between the two groups. The time spent for anesthesia was not included in operation time. We also compared resection efficiency, determined by counting the total numbers of resected and positive lymph nodes.
Preoperative and postoperative serum Tg concentrations were also checked. Postoperatively, all patients had routine follow-up with neck ultrasound and serum Tg concentration in an out-patient clinic for detection of recurrence or persistence of PTC. Within two months of the surgery, serum Tg concentrations were measured in T4-off (thyroid-stimulating hormone [TSH]-stimulated) states and, after that, routine measurements were performed every 6 to 12 months in a T4-on (TSH-suppressed) state. Serum TSH and anti-Tg antibodies were measured concurrently. We considered disease-free serum Tg concentrations to be less than 2 ng/mL in a T4-off state and less than 1 ng/mL in a T4-on state, based on the consensus recommendations of Mazzaferri et al. [20].
Statistical analyses between the groups were performed using independent t-tests, Mann-Whitney U tests and Fisher exact tests, as warranted.
Of the 53 patients, 43 were female (male to female ratio, 1:4.3), and the mean patient age was 46.9 years (range, 23 to 69 years). All the patients had a previous history of total thyroidectomy and central compartment node dissection. The mean number of previous operations was 1.3 (range, 1 to 2) and the mean duration from the most recent to the current operation was 66.0 months (range, 7 to 158 months).
We found that operation time was significantly shorter in patients evaluated by on-site UGL (group 2) than in those who were not (group 1) (102.0 minutes vs. 62.4 minutes, P < 0.001). The mean size of the resected lymph node was smaller in group 2 patients than in group 1 (1.0 cm vs. 0.8 cm, P = 0.013). Resection failure (1 patient) and postoperative complications (transient hypocalcemia, 1 patient) each occurred in one of the 32 patients in group 1. However, there were no significant differences between the groups in terms of resection success rate, rate of complications, and length of postoperative hospital stay (Table 1).
The mean number of resected lymph nodes was higher in group 1 than group 2 (3.56 vs. 3.19). However, the mean number of positive nodes was lower in group 1 than in group 2 (1.63 vs. 2.29), making the rate of positive node occurrence significantly higher in group 2 (51.2 vs. 82.7%, P < 0.001) (Table 2).
As mentioned above, neck ultrasound and serum Tg concentration measurement were performed for postoperative follow-up. During a mean follow-up of 27.6 months, there was only one patient in group 1 who showed sustained, suspicious lesions on neck ultrasound (the patient who failed in resection) whereas no patients in group 2 showed abnormal findings on ultrasound. In terms of serum Tg concentration, 28 patients (28/32) in group 1 and 19 patients (19/21) in group 2 showed less than 2 ng/mL in a T4-off state. At the last follow-up, 29 patients in group 1 and 20 patients in group 2 showed less than 1 ng/mL in a T4-on state (Fig. 2). There were no significant differences between the groups in the number of patients with tolerable serum Tg concentration in the postoperative period (Table 3). None of the patients showed elevated serum levels of anti-Tg antibodies.
Recurrences of PTC are not uncommon even after curative surgery. Postoperative follow-up with high-resolution ultrasound, combined with measurements of serum Tg concentrations, can increase the rates of detection of preclinical recurrences [3-8]. In contrast to locoregional recurrence of other malignancies, most locoregional recurrences of PTC can be controlled by proper surgical resection, with excellent patient prognosis [1-3,9].
The optimal management of preclinical nodal recurrences of PTC is unclear. The choice between simple observation and surgical removal remains a significant dilemma for clinicians [10-12]. Decisions should be made specific to individual patients, with long-term follow-up required to assess the outcomes of surgical management. Once they become aware of a recurrence of malignancy, however, most patients want these lesions to be removed as soon as possible.
In this study, all patients had previously undergone a total thyroidectomy with central neck compartment dissection. To avoid unnecessarily wide dissections and to minimize operative morbidities, we usually selectively resect nodal recurrences after on-site UGL. Indeed, although only 21 of the patients in this study were evaluated by on-site UGL, it proved useful, not only in reducing the length of surgery, but in enabling surgeons to perform more focused, accurate resections of nodal recurrences - even those smaller in size. By minimizing unnecessary dissections, limited, focused resections of recurrent foci can reduce postoperative morbidities [11,12]. Moreover, on-site UGL has several benefits compared to other localization methods. First, the operating surgeon can directly detect the location of lymph nodes just before the operation, resulting in more accurate resection with a more focused approach. Second, there is no need to arrange or organize preoperative localization procedures. By enabling the surgeon to localize lesions, UGL can eliminate the need for other, more time-consuming procedures and shorten bothersome preoperative localization procedures. Third, this technique is easy to perform and does not take much time (less than 5 minutes in our experience). Finally, the injected methylene blue dye can easily detect recurrent lymph nodes, allowing their effective removal while avoiding unnecessary dissection. Also, by staining these lesions, surgeons can be confident of their successful removal. In our experience, accurately injected methylene blue remains in the recurrent nodes for a sufficient period of time (more than 30 to 40 minutes) during surgery.
Methylene blue is widely used material in medical fields. When injected intraveneously, it has a half-life of 5.25 hours [21]. In surgical fields, methylene blue is used for lymph node staining of thyroid, breast cancer via direct or peritumoral injection, and used in parathyroidectomy with intravenous infusion [22-24]. Methylene blue is also known as an option in the treatment of ifosfamide-induced encephalopathy. In patients without predisposing risk factors like serotonergic psychiatric medications, use of methylene blue for lymph node staining is known as relatively safe [25,26].
In this study, there were some patients who showed elevated serum Tg concentrations even after successful resection (6 patients showed >2 ng/mL in T4-off state and 4 patients showed >1 ng/mL in T4-on state) and we could not find any abnormal/suspicious lesions in routine examinations, including neck ultrasound. The trouble was, even after performing additional imaging studies including radioactive iodine scans and PET-CT scans, no obvious foci suggesting recurrence or persistence of PTC were identified in these patients. As a last resort, we are conducting more frequent/close follow-up for those patients suspected of residual or recurrent disease in the subclinical levels.
In conclusion, on-site UGL technique performed by a surgeon is useful and effective for the resection of recurrent impalpable lymph nodes in patients with PTC.
Figures and Tables
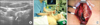 | Fig. 1(A) Transverse ultrasound image of a nodal recurrence (arrow) in central neck, (B) a patient undergoing on-site ultrasound-guided localization of recurrent lymph nodes after direct injection of methylene blue dye, and (C) a blue-stained lymph node in operative field (arrow). C, common carotid artery; T, trachea. |
 | Fig. 2Preoperative (Pre-op) and last follow-up (F/U) serum thyroglobulin (Tg) concentration of (A) group 1 (32 patients) and (B) group 2 (21 patients). Both levels were measured in T4-on states. There were 3 patients in group 1 and 1 patient in group 2 who showed serum Tg concentrations >1 ng/mL in last follow-up. |
References
1. Caron NR, Clark OH. Well differentiated thyroid cancer. Scand J Surg. 2004; 93:261–271.
2. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998; 338:297–306.
3. Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001; 86:1447–1463.
4. Cobin RH, Gharib H, Bergman DA, Clark OH, Cooper DS, Daniels GH, et al. American Association of Clinical Endocrinologists. American College of Endocrinology. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma. Endocr Pract. 2001; 7:202–220.
5. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006; 16:109–142.
6. Schlumberger M, Pacini F, Wiersinga WM, Toft A, Smit JW, Sanchez Franco F, et al. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol. 2004; 151:539–548.
7. Frasoldati A, Pesenti M, Gallo M, Caroggio A, Salvo D, Valcavi R. Diagnosis of neck recurrences in patients with differentiated thyroid carcinoma. Cancer. 2003; 97:90–96.
8. Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003; 88:3668–3673.
9. Goretzki PE, Simon D, Frilling A, Witte J, Reiners C, Grussendorf M, et al. Surgical reintervention for differentiated thyroid cancer. Br J Surg. 1993; 80:1009–1012.
10. Gemsenjager E, Perren A, Seifert B, Schuler G, Schweizer I, Heitz PU. Lymph node surgery in papillary thyroid carcinoma. J Am Coll Surg. 2003; 197:182–190.
11. Cross S, Wei JP, Kim S, Brams DM. Selective surgery and adjuvant therapy based on risk classifications of well-differentiated thyroid cancer. J Surg Oncol. 2006; 94:678–682.
12. Alzahrani AS, Raef H, Sultan A, Al Sobhi S, Ingemansson S, Ahmed M, et al. Impact of cervical lymph node dissection on serum TG and the course of disease in TG-positive, radioactive iodine whole body scan-negative recurrent/persistent papillary thyroid cancer. J Endocrinol Invest. 2002; 25:526–531.
13. Chao TC, Jeng LB, Lin JD, Chen MF. Reoperative thyroid surgery. World J Surg. 1997; 21:644–647.
14. Dralle H, Sekulla C, Haerting J, Timmermann W, Neumann HJ, Kruse E, et al. Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery. 2004; 136:1310–1322.
15. Salvatori M, Rufini V, Reale F, Gajate AM, Maussier ML, Revelli L, et al. Radio-guided surgery for lymph node recurrences of differentiated thyroid cancer. World J Surg. 2003; 27:770–775.
16. Karwowski JK, Jeffrey RB, McDougall IR, Weigel RJ. Intraoperative ultrasonography improves identification of recurrent thyroid cancer. Surgery. 2002; 132:924–928.
17. Kang TW, Shin JH, Han BK, Ko EY, Kang SS, Hahn SY, et al. Preoperative ultrasound-guided tattooing localization of recurrences after thyroidectomy: safety and effectiveness. Ann Surg Oncol. 2009; 16:1655–1659.
18. Triponez F, Poder L, Zarnegar R, Goldstein R, Roayaie K, Feldstein V, et al. Hook needle-guided excision of recurrent differentiated thyroid cancer in previously operated neck compartments: a safe technique for small, nonpalpable recurrent disease. J Clin Endocrinol Metab. 2006; 91:4943–4947.
19. McCoy KL, Yim JH, Tublin ME, Burmeister LA, Ogilvie JB, Carty SE. Same-day ultrasound guidance in reoperation for locally recurrent papillary thyroid cancer. Surgery. 2007; 142:965–972.
20. Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003; 88:1433–1441.
21. Peter C, Hongwan D, Kupfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000; 56:247–250.
22. Fukui Y, Yamakawa T, Taniki T, Numoto S, Miki H, Monden Y. Sentinel lymph node biopsy in patients with papillary thyroid carcinoma. Cancer. 2001; 92:2868–2874.
23. Tuttle TM. Technical advances in sentinel lymph node biopsy for breast cancer. Am Surg. 2004; 70:407–413.
24. Patel HP, Chadwick DR, Harrison BJ, Balasubramanian SP. Systematic review of intravenous methylene blue in parathyroid surgery. Br J Surg. 2012; 99:1345–1351.
25. Shah-Khan MG, Lovely J, Degnim AC. Safety of methylene blue dye for lymphatic mapping in patients taking selective serotonin reuptake inhibitors. Am J Surg. 2012; 204:798–799.
26. Gillman PK. CNS toxicity involving methylene blue: the exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity. J Psychopharmacol. 2011; 25:429–436.




 ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download