Abstract
Purpose
The empirical use of a chemotherapy regimen shows different results in individual breast cancer patient treatment. Recent studies showed the effectiveness of the adenosine triphosphate-based chemotherapy response assay (ATP-CRA). However, little is known about the correlation between chemosensitivity and breast cancer molecular subtypes. Therefore, we investigated whether the result of ATP-CRA is associated with a molecular subtype of breast cancer.
Methods
Two hundred eighty-seven patients diagnosed with breast cancer and receiving ATP-CRA at Mokdong Hospital, Ewha Womans University between September 2007 and December 2010 were enrolled in this study. Hormone receptor status, HER2/neu expression, and results of chemosensitivity tests of the patients was analyzed.
Results
In all of four subtypes, the combination of two agents showed significant higher mean cell death rate than a single agent. Within the breast cancer cell lines in this study, the range of chemosensitivity response was very wide and varied for each patient. For this reason, the molecular subtype of breast cancer is inconclusive in choosing an effective chemotherapeutic agent and in vitro chemosensitivity test, prior to therapy, could be a useful method for planning chemotherapy for each patient.
Conclusion
Chemosensitivity response to anticancer agents was found to vary depending on the individual breast cancer patients. The molecular subtype of breast cancer is inconclusive to choose the effective chemotherapeutic agent and the in vitro chemosensitivity test, prior to therapy, could be more useful for planning chemotherapy for each patient.
Breast cancer incidence rates have been gradually increasing worldwide for the past few decades and it is the second most common cancer in Korean women [1,2].
Surgical treatment is used as a main therapeutic modality in the treatment of breast cancer patients; also, systemic chemotherapy plays a key role in improvement of patients' survival. However, empirical use of a chemotherapy regimen shows different treatment results in individual patients. It may be due to heterogeneity of breast cancer. Therefore, several studies have been conducted to find available methods for predicting tumor response to chemotherapy [3-8]. However, these tests have limitations and are not widely used in clinical practice. These limitations are the low success rates in primary cell culture and requirement of large amount of samples. On the other hand, the adenosine triphosphate-based chemotherapy response assay (ATP-CRA) requires only a small number of cells, has a high success rate in primary cell culture and can be performed quickly [9-11]. Also, in our previous study, it showed 78.6% sensitivity, 100% specificity and 85.0% diagnostic accuracy [12]. Moreover, several studies suggest that ATP-CRA is a useful method for predicting chemotherapy response in ovarian cancer [13], lung cancer [14], gastric cancer [15], and colon cancer [16].
Breast cancer subtypes classified according to the expression of hormone receptors and HER2/neu over-expression are known to have different prognostic results. And, triple negative breast cancer showed relatively more sensitive to chemotherapy. Overexpression of HER2/neu is known to predict a poor outcome for chemotherapy [17]. However, little is known about the correlation between chemosensitivity and breast cancer molecular subtypes.
Within such a context, we aimed to investigate whether the results of ATP-CRA is associated with molecular subtype of breast cancer.
Two hundre and ninety patients diagnosed with breast cancer with ATP-CRA performed at Mokdong Hospital, Ewha Womans University between September 2007 and December 2010 were enrolled in this study. All patients received chemotherapy as an adjuvant. Among them, three patients diagnosed with ductal carcinoma in situ were excluded. All of the final enrolled patients were diagnosed as having invasive ductal carcinoma by a pathologist.
Tumor tissues were stored in Hank's Balanced Salt Solution (Gibco BRL, San Diego, CA, USA), containing 100 IU/mL penicillin (Sigma-Aldrich Co., St. Louis, MO, USA), 100 µg/mL streptomycin (Sigma-Aldrich Co.), 100 µg/mL gentamicin (Gibco BRL), 2.5 µg/mL amphotericin B (Gibco BRL) and 5% fetal bovine serum (FBS, Gibco BRL). These tissues underwent washing, quantification and mincing, followed by incubation with extracellular matrix degrading enzymes, such as dispase (Sigma-Aldrich Co.), pronase (Sigma-Aldrich Co.) and DNase (Sigma-Aldrich Co.), at 37℃ for 12-16 hours. Cells were harvested using a cell strainer (BD, Franklin Lakes, NJ, USA). To eliminate normal cells, the cell suspensions were subjected to ficoll (1.077 g/mL) gradient centrifugation, at 400 g for 15 minutes, and anti-CD45 antibody conjugated magnetic beads (Miltenyi Biotech, Auburn, CA, USA). The viability of isolated cells was tested using trypan blue exclusion.
Separated tumor cells were diluted to 2,000-20,000 viable cells/100 µL using Iscove's Modified Dulbecco's Medium (IMDM) (Gibco BRL), including 10% FBS, and seeded in triplicate to a 96-well ultra low attachment microplate (Costar, Cambridge, MA, USA), which was able to restrict growing normal cells, such as fibroblasts. In the treated groups, 100 µL of chemotherapeutic agents were added on the seeded cells and cultured for 48 hours in a CO2 incubator. In the untreated control groups, 100 µL of IMDM, without chemotherapeutic agents, was added to 3-6 wells of the microplate. For the purpose of quality control, a negative control group of 3-6 wells (only seeding medium without cells) and two positive control groups were included in the culture plate. Each positive control group was composed of 3 wells that contained the minimal (105 pg ATP) and median (280 pg ATP) amounts of ATP, as measured in 1,000 tumor cells harvested from tissue. The final concentrations of anticancer drugs were determined by training set experiments, which exhibited scattered distribution of cell deaths from each specimen (data not shown); 5-fluorouracil (5-FU, 50 µg/mL), paclitaxel (8.5 µg/mL), docetaxel (3.7 µg/mL), doxorubicin (1.5 µg/mL), methotrexate (0.37 µg/mL) and cyclophosphamide (4.7 µg/mL). Cells from the untreated control and treated groups were lysed, and the amounts of ATP in the cell lysates measured using luciferin and excessive luciferase (Roche, Mannheim, Germany), followed by flash type luminescence measurements on a Victor 3 multilabel counter (PerkinElmer, Boston, MA, USA). The cell death rate for each drug was calculated as follows:
To calculate the intraassay mean coefficient of variation (CV) value, luminescence values of each specimen were measured 3 times. We then confirmed whether the measured values at 280 pg of ATP were higher than those at 105. If there were microorganism contamination, inadequate number of cells and an intraassay mean CV greater than 30, the test was considered a failure. If the measured values in the untreated control were lower than those in the positive group (105 pg of ATP), the specimen was considered to have unacceptable viability.
Expression of estrogen receptor (ER), progesterone receptor (Pg-R), erb-B2 was determined by immunostaining. Tissues for immunohistochemical assay were obtained from paraffin block of tru-cut biopsy or surgical resection specimens. Sections were cut from each block, dewaxed in xylene, and then hydrated using graded concentrations of ethanol in distilled water. Microwave antigen retrieval was performed before incubation with primary antibodies at room temperature for 5 minutes. Samples were incubated with each antibody at various dilutions. After incubation, specimens were processed by the avidin-biotin peroxidase complex method to detect protein accumulation. Immunostaining was performed using an automated staining system (Bond-X System; Vision Biosystems, Mount Waverley VIC, Australia). Expression of ER and Pg-R was reported as an expression rate and classed as negative, weak positive, intermediate positive and strong positive; Weak, intermediate and strong positive considered as positive. Expression of erb-B2 was categorized as 0, 1+, 2+, or 3+. Tumors were considered as HER2/neu negative by scoring 0 or 1+, whereas cased with score 3+ were considered HER2/neu positive. HER2/neu overexpression was confirmed by the fluorescence in situ hybridization technique for score 2+ in expression of erb-B2 cancer.
The statistical significance of any differences for the subtypes of breast cancer in ATP-CRA result to different cytotoxic drugs was confirmed using the one-way analysis of variance test. The post-hoc analysis was used to compare mean value between the two groups. P-values of <0.05 were considered statistically significant.
Two hundre eighty-seven patients were included in this study. All patients were female and their mean age was 48.9 ± 9.7 years. Ninety-three patients were stage I (32.1%), 123 patients were stage II (42.4%), 54 patients were stage III (18.6%), and 4 patients were stage IV (1.4%).
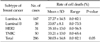
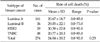
Patients were classified into 4 groups as luminal A (ER+ and/or PR+, HER2/neu-), luminal B (ER+ and/or PR+, HER2/neu+), HER2 (ER-, PR-, and HER2/neu+) and triple negative (triple negative breast cancer [TNBC], ER-, PR-, HER2/neu-). There were 167 patients in luminal A, 38 in luminal B, 31 in HER2 and 50 in triple negative breast cancer (Table 1).
The lists of tested agents and their corresponding results are presented in Table 2. Sensitivity rates ranged from 0% to 97.0%. The combination of two agents showed a higher mean cell death rate than a single agent. Among single agents, 5-FU showed the highest mean cell death rate.
We evaluated whether chemosensitivity is different between breast cancer subtypes. We conducted the study in some single agent regimens and combination regimens (combination regimens: doxorubicin and docetaxel, doxorubicin and paclitaxel, doxorubicin and cyclophosphamide, single agent regimens: 5-FU, docetaxel.) that showed high mean cell death rate (Tables 3, 4, 5, 6, 7). There was no significant difference between four of the breast cancer subtypes in all of the regimens. In combination regimens of doxorubicin and docetaxel, doxorubicin and paclitaxel, also in combination of doxorubicin and cyclophosphamide, TNBC group showed the highest cell death rate and there was only significant difference between luminal A and TNBC. In 5-FU, HER2 group showed the highest cell death rate while there was only significant difference between luminal A and HER2. With the single agent of docetaxel, HER2 group showed the highest cell death rate but there was no significant difference between all four groups. We also evaluated the correlation in the other agents and there was also no significant difference between the four groups in cell death rate of all agents (data not shown).
In each other breast cancer molecular subtype, we analyzed whether mean cell death rate of chemotherapy agents is different (Figs. 1, 2, 3, 4). In all of four subtypes, combinations of two agents showed significant higher mean cell death rate than a single agent. Otherwise, in combination or single agent therapy, there's no significant difference between the kinds of chemotherapeutic agents. In all of four groups, combination of doxorubicin and docetaxel showed the highest mean cell death rate.
The development of chemotherapy improved the survival of breast cancer patients. However, the response to chemotherapy is very different in patients. Breast cancer is a heterogeneous disease; therefore tumors with similar stage and clinicopathologic characteristics are diverse in disease behavior and response to treatment and outcome. For this reason, a considerable number of patients treated with empirical chemotherapeutic regimens do not benefit from adjuvant therapy. Several studies have been undertaken to find methods capable of predicting tumor response to chemotherapy or helpable for choosing chemotherapeutic agents. Although some biologic markers have shown potential predictive ability, it is not clearly accepted which biologic markers helpfully predict response to chemotherapy in individual patients. In previous studies, chemosensitivity tests were considered one of the viable methods that predict tumor response to chemotherapy and are useful in choosing the chemotherapy agents [12-16].
The ATP-CRA assay requires only a small number of cells, can be performed quickly, and this method includes the suppression of elimination of normal cells from tissue specimens. Also, it is considered more accurate than other reported chemosensitivity tests, such as human tumor clonogenic assay, the thymidine incorporation assay, the histoculture drug-response assay and the 3-(4,5-dimethylthiazol-2-yl)-2, 5-siphenyltetrazolium bromide assay [5,8,18,19].
In the present study, the highest mean cell death rate was observed in doxorubicin combined with docetaxel and the combined regimens were superior to single agent regimens. Within single agents, 5-FU showed the highest mean cell death rate. The rank of agents is different in studies [12,20,21]. However, the results of mean cell death rate were very close to each other.
Biologic markers of breast cancer, especially hormone receptors-ER, PR- and HER2/neu, are used as prognostic factors and frequently studied as predictors of chemosensitivity to various drugs [22-24]. As predictors of chemosensitivity, however, the clinical significance of biologic markers is still controversial. In our previous study, the expression of ER or PR significantly predicted tumor response but their diagnostic accuracy was low [12]. Also, their correlation with chemosensitivity tests is not clear.
In our analysis, all combination or single agent regimens showed higher mean cell death rate in TNBC than the other molecular subtypes, except 5-FU. The statistical significance, however, was limited. In all molecular subtypes, the combination of two chemotherapeutic agents showed significant superior cell death rate to single agents but there's no significant difference according to the kinds of combination agents. Woo et al. [20] observed that gemcitabine was more effective in HER2/neu over-expressed breast cancer than for negative HER2/neu breast cancer. And Pritchard et al. [25] reported that amplification of HER2/neu in breast cancer is associated with responsiveness to anthracyclin-containing chemotherapeutic agents. Other investigators, however, reported that the response to the chemotherapeutic agents did not correlate with the expression of biologic markers, including HER2/neu, p53 and ER [3,26].
Also, in this study, molecular subtype was used for classification of patients. Molecular subtype consists of combinations of hormone receptors and HER2/neu amplification status. Because of that, the results of analysis could be different from the other studies that analyzed single factors of hormone receptors or HER2/neu amplification status. In the previous studies, molecular subtypes were strongly correlated with prognosis of breast cancer patients. Investigators noted that molecular subtypes are even better than traditional histopathologic parameters as a predictor for short-term prognosis of breast cancer patients [27,28]. In addition, response to the chemotherapy was different in molecular subtypes [29]. In this context, the analysis of correlation between molecular subtype of breast cancer and chemosensitivity could be a meaningful study. This study however did not show correlation of chemosensitivity to the chemotherapeutic agents and the molecular subtypes of breast cancer. However, there was significant difference when TNBC and HER2 subtypes were compared with luminal A. This result is consistant with other previous reports in which the TNBC and HER2 subtypes had better chemosensitivity than the luminal subtype [30,31]. We supposed that higher chemosensitivity of TNBC and HER2 subtype is due to their high proliferative activity. The triple negative and HER2+ breast cancer subtypes are characterized by high expression of the proliferation cluster of genes [32]. The higher expression of genes regulating proliferation was shown to predict pathologic complete response to neoadjuvant chemotherapy [33], and it supports the relationship of proliferation to chemosensitivity.
The molecular subtypes were not helpful factors for choosing the specific chemotherapeutic agents. We supposed that the negative result of this study is caused by heterogeneity of the breast cancer. Even though tumors belong to the same molecular subtype, individual tumors have distinctive behavior and responsiveness to chemotherapy. As a result, a single factor, including molecular subtype, may be difficult in helping select chemotherapeutic agents.
Our study was a retrospective study and conducted in a single center. For this reason, there is a limitation in the results of this study.
In conclusion, the chemosensitivity response to anticancer agents was found to vary depending on the individual breast cancer patients. In all molecular types, combination regimens were more cytotoxic in vitro than the single agent regimens. Also, most cytotoxic agents were more effective in triple negative breast cancer group. In the breast cancer cell lines in this study, the range of chemosensitivity response was very wide and varied for each patient. For this reason, the molecular subtype of breast cancer is inconclusive in choosing an effective chemotherapeutic agent and the in vitro chemosensitivity test, prior to therapy, could be more useful for planning chemotherapy for each patient. In the future, a randomized prospective study with a large cohort is required to confirm the results of this study.
Figures and Tables
Fig. 1
The in vitro adenosine triphosphate-based chemosensitivity test of the cultured breast cancer cell lines for the molecular subtype of luminal A. 5-FU, 5-fluorouracil; CPM, cyclophosphamide.

Fig. 2
The in vitro adenosine triphosphate-based chemosensitivity test of the cultured breast cancer cell lines for the molecular subtype of luminal B. 5-FU, 5-fluorouracil; CPM, cyclophosphamide.

Fig. 3
The in vitro adenosine triphosphate-based chemosensitivity test of the cultured breast cancer cell lines for the molecular subtype of HER2. 5-FU, 5-fluorouracil; CPM, cyclophosphamide.

Fig. 4
The in vitro adenosine triphosphate-based chemosensitivity test of the cultured breast cancer cell lines for the molecular subtype of triple negative breast cancer. 5-FU, 5-fluorouracil; CPM, cyclophosphamide.

Table 2
The results of in vitro adenosine triphosphate-based chemosensitivity test of the cultured breast cancer cell lines to the cytotoxic agents

Table 3
The results of in vitro adenosine triphosphate-based chemosensitivity test of the cultured breast cancer cell lines to chemotherapeutic regimen of doxorubicin combined with docetaxel
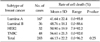
Table 4
The results of in vitro adenosine triphosphate-based chemosensitivity test of the cultured breast cancer cell lines to chemotherapeutic regimen of doxorubicin combined with paclitaxel.
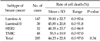
Table 5
The results of in vitro adenosine triphosphate-based chemosensitivity test of the cultured breast cancer cell lines to chemotherapeutic regimen of doxorubicin combined with cyclophosphamide
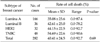
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
2. Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2008. Goyang: Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center;2010.
3. Takamura Y, Kobayashi H, Taguchi T, Motomura K, Inaji H, Noguchi S. Prediction of chemotherapeutic response by collagen gel droplet embedded culture-drug sensitivity test in human breast cancers. Int J Cancer. 2002; 98:450–455.
4. Kern DH, Drogemuller CR, Kennedy MC, Hildebrand-Zanki SU, Tanigawa N, Sondak VK. Development of a miniaturized, improved nucleic acid precursor incorporation assay for chemosensitivity testing of human solid tumors. Cancer Res. 1985; 45(11 Pt 1):5436–5441.
5. Tanigawa N, Morimoto H, Dohmae N, Shimomatsuya T, Takahashi K, Muraoka R. In vitro growth ability and chemosensitivity of gastric and colorectal cancer cells assessed with the human tumour clonogenic assay and the thymidine incorporation assay. Eur J Cancer. 1992; 28:31–34.
6. Wilbur DW, Camacho ES, Hilliard DA, Dill PL, Weisenthal LM. Chemotherapy of non-small cell lung carcinoma guided by an in vitro drug resistance assay measuring total tumour cell kill. Br J Cancer. 1992; 65:27–32.
7. Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res. 1998; 4:389–398.
8. Furukawa T, Kubota T, Hoffman RM. Clinical applications of the histoculture drug response assay. Clin Cancer Res. 1995; 1:305–311.
9. Sevin BU, Peng ZL, Perras JP, Ganjei P, Penalver M, Averette HE. Application of an ATP-bioluminescence assay in human tumor chemosensitivity testing. Gynecol Oncol. 1988; 31:191–204.
10. Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995; 55:5276–5282.
11. Konecny G, Crohns C, Pegram M, Felber M, Lude S, Kurbacher C, et al. Correlation of drug response with the ATP tumorchemosensitivity assay in primary FIGO stage III ovarian cancer. Gynecol Oncol. 2000; 77:258–263.
12. Kim HA, Yom CK, Moon BI, Choe KJ, Sung SH, Han WS, et al. The use of an in vitro adenosine triphosphate-based chemotherapy response assay to predict chemotherapeutic response in breast cancer. Breast. 2008; 17:19–26.
13. O'Meara AT, Sevin BU. Predictive value of the ATP chemosensitivity assay in epithelial ovarian cancer. Gynecol Oncol. 2001; 83:334–342.
14. Moon YW, Sohn JH, Kim YT, Chang H, Jeong JH, Lee YJ, et al. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided versus empirical chemotherapy in unresectable non-small cell lung cancer. Anticancer Res. 2009; 29:4243–4249.
15. Kawamura H, Ikeda K, Takiyama I, Terashima M. The usefulness of the ATP assay with serum-free culture for chemosensitivity testing of gastrointestinal cancer. Eur J Cancer. 1997; 33:960–966.
16. Huh JW, Park YA, Sohn SK, Choi SH. In-vitro Chemosensitivity test for colorectal cancer using an adenosine-triphosphate-based chemotherapy response assay (ATP-CRA). J Korean Soc Coloproctol. 2007; 23:172–179.
17. Allred DC, Clark GM, Tandon AK, Molina R, Tormey DC, Osborne CK, et al. HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol. 1992; 10:599–605.
18. Maehara Y, Anai H, Tamada R, Sugimachi K. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. Eur J Cancer Clin Oncol. 1987; 23:273–276.
19. Petty RD, Sutherland LA, Hunter EM, Cree IA. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J Biolumin Chemilumin. 1995; 10:29–34.
20. Woo SU, Bae JW, Kim HG, Choi SH, Kang DH, Lee JB, et al. Correlation between the in vitro ATP-based chemosensitivity assay and HER2/neu expression in women with breast cancer. J Int Med Res. 2007; 35:753–761.
21. Choi SK, Jeong J, Lee SA, Hwang SH, Ahn SG, Jung WH, et al. Heterogeneous chemosensitivity of breast cancer determined by adeonsine triphosphate based chemotherapy response assay. J Breast Cancer. 2010; 13:180–186.
22. Charfare H, Limongelli S, Purushotham AD. Neoadjuvant chemotherapy in breast cancer. Br J Surg. 2005; 92:14–23.
23. Ogston KN, Miller ID, Schofield AC, Spyrantis A, Pavlidou E, Sarkar TK, et al. Can patients' likelihood of benefiting from primary chemotherapy for breast cancer be predicted before commencement of treatment? Breast Cancer Res Treat. 2004; 86:181–189.
24. Hannemann J, Oosterkamp HM, Bosch CA, Velds A, Wessels LF, Loo C, et al. Changes in gene expression associated with response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2005; 23:3331–3342.
25. Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006; 354:2103–2111.
26. Rozan S, Vincent-Salomon A, Zafrani B, Validire P, De Cremoux P, Bernoux A, et al. No significant predictive value of c-erbB-2 or p53 expression regarding sensitivity to primary chemotherapy or radiotherapy in breast cancer. Int J Cancer. 1998; 79:27–33.
27. Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. 2009; 20:628–635.
28. Zaha DC, Lazar E, Lazureanu C. Clinicopathologic features and five years survival analysis in molecular subtypes of breast cancer. Rom J Morphol Embryol. 2010; 51:85–89.
29. Chen XS, Wu JY, Huang O, Chen CM, Wu J, Lu JS, et al. Molecular subtype can predict the response and outcome of Chinese locally advanced breast cancer patients treated with preoperative therapy. Oncol Rep. 2010; 23:1213–1220.
30. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005; 11:5678–5685.
31. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007; 13:2329–2334.
32. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–10874.
33. Gianni L, Zambetti M, Clark K, Baker J, Cronin M, Wu J, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005; 23:7265–7277.




 ePub
ePub Citation
Citation Print
Print


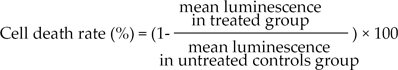

 XML Download
XML Download