Abstract
Purpose
Hernia repairs are the most common elective abdominal wall procedures performed by general surgeons. The use of a mesh has become the standard for hernia repair surgery. Herein, we discuss a management strategy for chronic mesh infections following open inguinal hernia repair with onlay prosthetic mesh.
Methods
In this study, 15 patients with chronic mesh infections following open inguinal hernia repairs were included. The medical records of these patients were retrospectively reviewed and information regarding presentation, type of previous hernia repair, type of mesh, operative findings and bacteriological examination results were obtained. In all cases, the infected mesh was removed completely and the patients were treated with antibiotic regimens and local wound care.
Results
Fifteen mesh removals due to chronic infection were performed between January 2000 and March 2012. The mean interval of hernia repair to mesh removal was 49 months. All patients were followed up for a median period of 62 months (range, 16 to 115 months). In all patients, the infections were resolved successfully and none were persistent or recurrent. However, one patient developed recurrent hernia and one developed nerve injury.
Hernia repairs are the most common elective abdominal wall procedures performed by general surgeons. Recently, the use of a mesh has become the standard in hernia repair surgery worldwide owing to the reduced rates of recurrence and technical ease of the operation [1-3]. However, mesh-related complications have become increasingly more frequent. Postsurgical mesh-related infections are rare but troublesome complications that cause considerable morbidity and necessitate mesh removal. Antibiotics and mesh-saving operations are not sufficient to eradicate the infection in the majority of cases [4-7].
However, the true incidence of chronic mesh infection following inguinal hernia repair is unclear. Gilbert and Felton [8] reported an infection rate of 0.8% in a review of 1,834 mesh inguinal hernia repairs, whereas the pooled Lichtenstein series [9] reported an overall infection rate of 0.003% for patch repairs of inguinal hernias. Clearly, the incidence of chronic mesh infection is highly variable among published series and might be related to the surgical technique, type of mesh, and strategies necessary to prevent infections. The main approach to prevent mesh infection is intravenous preoperative administration of antimicrobial agents. However, controversy exists concerning the use of antibiotic prophylaxis in hernia surgery [10]. We conducted two randomized trials in our clinic to investigate the use of antibiotic prophylaxis in open prosthetic inguinal hernia repair and found a major advantage with prophylactic antibiotic use with surgical site infection rates of 1.4 and 0.9% [11,12].
Herein, we report our series of chronic mesh infection following open inguinal hernia repair with onlay prosthetic mesh and our treatment of this complication.
We conducted a retrospective review of chronic mesh infections following inguinal hernia repair managed at the Ankara University School of Medicine, Department of General Surgery, Hernia Surgery Unit (Ankara, Turkey) between January 2000 and March 2012. The medical records of these patients were reviewed retrospectively and information regarding presentation, types of previous hernia repair, types of mesh used, operative findings and bacteriological examination results were obtained. Patients who underwent an open hernia repair using multifilament polypropylene mesh and the onlay technique were evaluated. The majority of patients in this series had hernia repairs at other institutions and were referred to our clinic for treatment of chronic infections. Infections that occurred 30 days after operation and involved only skin or subcutaneous tissue of the incision were defined as superficial incisional infections [13]. Patients presenting with superficial incisional infections were excluded and only those diagnosed with deep prosthetic infections were included in the present study.
All of the referred patients had received repeated courses of antibiotic therapy before admission to our clinic. Our treatment strategy includes systemic antibiotic therapy, drainage of abscesses and removal of the infected mesh. In all of the patients, the infected meshes were removed completely completely and associated sinus tracts were extirpated. Specimens were sent for bacteriological examination at the time of the operation. In none of the patients did we attempt to reinforce the transversalis fascia, which was thickened and fibrosed even after mesh removal. All operations were performed by a consultant surgeon or under direct supervision of the consultant. Patients were further followed up and reviewed in an outpatient clinic to determine hernia recurrence following mesh removal.
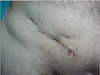
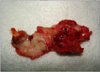
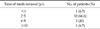
In our clinic, between the years 2000 and 2012, 2,940 hernia repairs were performed and in 4 of these patients, graft infection is observed. Eleven of these patients who had graft infection, had hernia surgery in other medical centers. During the study period, 15 mesh removals were performed because of chronic infections in 14 males and one female with a median age of 52 years (range, 35 to 75 years). At the time of presentation, 13 patients had chronic sinus discharge while two had abscesses (Fig. 1). Systemic manifestations of infection were not observed in any of the patients. The interval from hernia repair to mesh removal was 4 to 204 months (Table 1, Fig. 2). During this period, all patients were treated with antibiotic regimens, abscess drainage and local wound care and were referred to our clinic after failure of this conservative approach.
Bacteriological examinations of the materials that were removed were negative in nine patients, whereas bacterial isolates were obtained in six patients. Four cultures were positive for Staphylococcus aureus, of which two were methicillin-resistant. One culture contained enteroccoci and Escherichia coli and another was positive for coagulase-negative Staphylococcus.
Patients were followed up for a median period of 62 months (range, 16 to 115 months). In all of the patients, infections were resolved successfully and none were persistent or recurrent. However, one patient reported a burning sensation (paresthesia) after mesh removal and developed nerve injury and while another patient, who underwent mesh removal 4 months after the initial hernia repair, developed a recurrent hernia during the follow-up period.
Open tension-free mesh repair is the gold standard for managing inguinal hernias. In spite of its advantages, the use of prosthetic mesh in hernia repair also increases the risk of complications, such as chronic infection. Deep prosthetic infections should be distinguished from superficial incisional infections [14,15], which tend to occur in the early postoperative period and do not seem to be influenced by the use of mesh. These infections are typically managed without the need for mesh removal since they do not involve the mesh. In contrast, deep prosthetic infections have been rarely reported in the literature. One of the most striking features of prosthetic infections is that they tend to present after a delayed period following mesh repair [4,16]. In our series, the mean time period between hernia repair and removal of the infected mesh was 49 months. In deep prosthetic infections, patients may present with local acute inflammation and systemic manifestations such as fever and malaise. More frequently, deep mesh infections tend to present in a more indolent manner with chronic, persistent or recurrent signs and symptoms. Typically, patients present with sinus formation as seen in our series [4,5,16].
Radiological techniques, including ultrasound and computerized tomography, are useful for diagnosis [17]; however, in our series, these techniques were not necessary for diagnosing mesh infection.
The most common pathogens involved in mesh infections are Staphylococcus species (especially S. aureus), Streptococcus spp., gram-negative bacteria (mainly Enterobacteriaceae) and anaerobic bacteria [4,16,18,19]. Methicillin-resistant S. aureus (MRSA) accounted for 63% of the isolated microorganisms in a study of mesh-related infections following incisional herniorrhaphy [20]. In the present study, bacterial isolates were obtained in 40% of the cases, from which 66% were MRSA, which is in agreement with previous reports [4,7,16]. Recent or concomitant antibiotherapy could have been responsible for the low identification of causative pathogens from intraoperative mesh samples of these patients. It is well known that Staphylococcus spp., which are the most common causative organisms in mesh infections, produce biofilms on prostheses, which also contributed to the low identification rate of organisms via bacteriological examinations [21]. Because culture results can be negative in many cases, a diagnosis of chronic mesh infection is based on clinical presentation.
A combined medical and immediate surgical approach involving intravenous antimicrobial agents and complete surgical removal of the mesh is suggested for mesh-related infections to reduce the risk of infection recurrence or severe complications, such as visceral adhesions and fistulae. Conservative surgical approaches such as abscess drainage, sinus excision or partial mesh excision can fail and result in recurrent mesh infections [7,18,22]. In the present series, all cases underwent mesh removal and all patient symptoms were resolved successfully with no persistent or recurrent infections.
Removal of the infected mesh may not result in recurrent herniation if sufficient fibrous scarring remains. The initial reaction in response to surgically implanted prosthetic mesh is characterized by acute inflammatory cell infiltration followed by fibroblast infiltration through the interstices of the porous mesh that gradually replace inflammatory cells [23-25]. Under ideal circumstances, implanted mesh material becomes incorporated into the surrounding tissues via fibrous infiltration and the formation of a linear neo-fascia. Recurrent hernia does not develop if adequate fibrous reaction and scarring occurred prior to mesh removal. Polypropylene mesh was used in all patients in the present series. Fibrosis at the site of mesh implantation mostly depends on the mesh structure and material. Despite numerous recent developments, polypropylene remains the most commonly used material for hernia repairs. Due to the large pore size and biocompatibility of the material, it has the greatest tissue in-growth and has been shown to excite a significant inflammatory reaction resulting in excessive scarring [26]. The strength of mesh repair lies in the fibrous reaction evoked by the prosthetic material rather than the mesh itself; therefore, mesh removal does not always result in hernia recurrence. To the best of our knowledge, there are no previous reports documenting hernia recurrence following removal of infected mesh material inserted by open or laparoscopic techniques [5,6]. Although recurrence is rare after mesh removal, recurrence was observed in one of the cases (6.7%) in the present series. This case was treated with a laparoscopic total extraperitoneal approach. The reason for this high recurrence rate may be associated with the limited number of patients. Laparoscopic total extraperitoneal hernia repair has been considered a safe and effective surgical approach for recurrent hernia following an anterior technique [27].
One limitation to our study was the limited number of cases. However, an infection following hernia repair is not a frequent complication; therefore, management of chronic mesh infections should rely on reports of series with a greater number of patients.
In conclusion, chronic mesh infections may present after a long period following hernia repair, which are diagnosed on clinical grounds. Complete mesh removal ensures infection eradication and rarely results in hernia recurrence if sufficient fibrous scarring remains.
Figures and Tables
ACKNOWLEDGEMENTS
The authors wish to extend their sincere gratitude to the staff and advisory Committee on Hospital Infection Control at Ankara University for assistance in completing this project.
References
1. Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000. 343:392–398.
2. Vrijland WW, van den Tol MP, Luijendijk RW, Hop WC, Busschbach JJ, de Lange DC, et al. Randomized clinical trial of non-mesh versus mesh repair of primary inguinal hernia. Br J Surg. 2002. 89:293–297.
3. Lichtenstein IL, Shulman AG, Amid PK, Montllor MM. The tension-free hernioplasty. Am J Surg. 1989. 157:188–193.
4. Taylor SG, O'Dwyer PJ. Chronic groin sepsis following tension-free inguinal hernioplasty. Br J Surg. 1999. 86:562–565.
5. Ismail W, Agrawal A, Zia MI. Fate of chronically infected onlay mesh in groin wound. Hernia. 2002. 6:79–81.
6. Avtan L, Avci C, Bulut T, Fourtanier G. Mesh infections after laparoscopic inguinal hernia repair. Surg Laparosc Endosc. 1997. 7:192–195.
7. Tolino MJ, Tripoloni DE, Ratto R, Garcia MI. Infections associated with prosthetic repairs of abdominal wall hernias: pathology, management and results. Hernia. 2009. 13:631–637.
8. Gilbert AI, Felton LL. Infection in inguinal hernia repair considering biomaterials and antibiotics. Surg Gynecol Obstet. 1993. 177:126–130.
9. Shulman AG, Amid PK, Lichtenstein IL. The safety of mesh repair for primary inguinal hernias: results of 3,019 operations from five diverse surgical sources. Am Surg. 1992. 58:255–257.
10. Deysine M. Pathophysiology, prevention, and management of prosthetic infections in hernia surgery. Surg Clin North Am. 1998. 78:1105–1115.
11. Kuzu MA, Hazinedaroglu S, Dolalan S, Ozkan N, Yalcin S, Erkek AB, et al. Prevention of surgical site infection after open prosthetic inguinal hernia repair: efficacy of parenteral versus oral prophylaxis with amoxicillin-clavulanic acid in a randomized clinical trial. World J Surg. 2005. 29:794–799.
12. Yerdel MA, Akin EB, Dolalan S, Turkcapar AG, Pehlivan M, Gecim IE, et al. Effect of single-dose prophylactic ampicillin and sulbactam on wound infection after tension-free inguinal hernia repair with polypropylene mesh: the randomized, double-blind, prospective trial. Ann Surg. 2001. 233:26–33.
13. National Surgical Quality Improvement Program. Noncardiac terms and definitions, revisions to definitions 9-1-95. National Surgical Quality Improvement Program. Managerial I site operations manual. St. Louis Continuing Education Center;4/7–4/26.
14. Pierce RA, Spitler JA, Frisella MM, Matthews BD, Brunt LM. Pooled data analysis of laparoscopic vs. open ventral hernia repair: 14 years of patient data accrual. Surg Endosc. 2007. 21:378–386.
15. Taylor EW, Duffy K, Lee K, Hill R, Noone A, Macintyre I, et al. Surgical site infection after groin hernia repair. Br J Surg. 2004. 91:105–111.
16. Fawole AS, Chaparala RP, Ambrose NS. Fate of the inguinal hernia following removal of infected prosthetic mesh. Hernia. 2006. 10:58–61.
17. Falagas ME, Kasiakou SK. Mesh-related infections after hernia repair surgery. Clin Microbiol Infect. 2005. 11:3–8.
18. Berliner SD. Clinical experience with an inlay expanded polytetrafluoroethylene soft tissue patch as an adjunct in inguinal hernia repair. Surg Gynecol Obstet. 1993. 176:323–326.
19. Bauer JJ, Salky BA, Gelernt IM, Kreel I. Repair of large abdominal wall defects with expanded polytetrafluoroethylene (PTFE). Ann Surg. 1987. 206:765–769.
20. Cobb WS, Harris JB, Lokey JS, McGill ES, Klove KL. Incisional herniorrhaphy with intraperitoneal composite mesh: a report of 95 cases. Am Surg. 2003. 69:784–787.
21. Reilly JS, Baird D, Hill R. The importance of definitions and methods in surgical wound infection audit. J Hosp Infect. 2001. 47:64–66.
22. Marrie TJ, Costerton JW. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol. 1984. 19:687–693.
23. Arnaud JP, Eloy R, Adloff M, Grenier JF. Critical evaluation of prosthetic materials in repair of abdominal wall hernias: new criteria of tolerance and resistance. Am J Surg. 1977. 133:338–345.
24. Bellon JM, Bujan J, Contreras L, Hernando A, Jurado F. Macrophage response to experimental implantation of polypropylene prostheses. Eur Surg Res. 1994. 26:46–53.
25. Tyrell J, Silberman H, Chandrasoma P, Niland J, Shull J. Absorbable versus permanent mesh in abdominal operations. Surg Gynecol Obstet. 1989. 168:227–232.
26. Amid PK. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia. 1997. 1:15–21.
27. Jang IS, Lee SM, Kim JH, Kim BS, Choi SI. Clinical usefulness of laparoscopic total extraperitoneal hernia repair for recurrent inguinal hernia. J Korean Surg Soc. 2011. 80:313–318.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download