Abstract
Purpose
Primary breast lymphoma is a very rare disease, accounting for 0.4-0.5% of all breast malignancies. Due to the rarity, there are only limited reports of this disease in Korean women. In this reason, we report the experience of a single institution in Korea with primary breast lymphoma (PBL).
Methods
We retrospectively reviewed the medical records of 9 patients with PBL and evaluated the clinicopathologic characteristics and treatment outcomes.
Results
All nine patients were female and had diffuse large B-cell lymphoma (DLBL). The median age at diagnosis was 47.9 years and the median tumor size was 3.8 cm in diameter. The most common symptom was a painless palpable mass. Five patients were classified as stage IEA and four patients were IIEA according to the Ann Arbor staging system. Four patients underwent excisional biopsy and one patient underwent a lumpectomy with sentinel lymph node biopsy due to uncertain histology of the preoperative core needle biopsy. Nine patients received anthracycline containing combined chemotherapy; among them, five patients were treated with a rituximab containing regimen. Four patients received radiotherapy combined with chemotherapy. A complete response was achieved in eight patients. During the 44 months of the median follow-up period, three cases of relapse occurred, and among them, two patients died due to disease progression.
Breast lymphoma is rare clinical entity. The disease may arise in both sexes, while it occurs almost exclusively in women. About 25-40% of non-Hodgkin's lymphoma (NHL) patients present with a primary extranodal origin [1,2], and the extranodal lymphoma could arise in almost every organ in the body [2,3]. However, because of paucity of the lymphoid tissue in the breast [4], primary breast lymphoma (PBL) and secondary involvement of the breast by lymphoma are rare [5,6].
PBL is diagnosed when the breast is the first site or major manifestation of the lymphoma, and there is no documentation of lymphoma elsewhere, except the ipsilateral axillary node [7]. PBL accounts for more than 40% of cases of breast lymphoma [8]. PBL has been rarely reported, and accounts for less than 1% of all NHL and 1.7-2.2% of extranodal NHL [1,9]. Diffuse large B-cell lymphoma (DLBL) is the most common histologic type of PBL, whereas low grade lymphomas, including mucosal-associated lymphoid tissue (MALT) lymphoma, marginal zone B-cell lymphoma and follicular lymphoma (FL), are the majority of disseminated lymphomas involving the breast [8]. Surgery, chemotherapy and/or radiotherapy, as either monotherapy or combined treatment have been reported as treatment modalities for PBL.
Due to the rarity of PBL, limited information about this disease in Korean women is available. Here, we report our experience of the clinicopathologic characteristics and treatment outcomes of this rare disease in our institution.
We retrospectively reviewed the electronic database of the Samsung Medical Center, Seoul, Korea, for the years between 1997 and 2009. Twenty three patients with infiltration of lymphoid malignant cells in the mammary tissue were identified. We adapted the original criteria of PBL by Wiseman and Liao [7], PBL was diagnosed when the patients fulfilled the following criteria: 1) technically adequate pathologic specimen, 2) close association of breast tissue and lymphomatous infiltration, 3) absence of previous extramammary lymphoma and 4) no evidence of widespread documentation of a similar histologic type of the lymphoma except in the ipsilateral axillary lymph nodes. Among the 23 patients, 9 women met the eligibility criteria for PBL, and a retrospective review was conducted.
All patients were diagnosed histologically by excisional biopsy or core needle biopsy. All pathologic specimens were reviewed by a pathologist, and the histologic type of lymphoma was classified according to the World Health Organization classification [10] based on morphologic examination of hematoxylin & eosin stain coupled with immunohistochemical stains. Patients were staged according to the Ann Arbor system [11], and the prognostic index was evaluated for all patients according to the International Prognostic Index (IPI) score [12]. Initial staging procedures included a complete blood count, chemistry, chest X-ray, mammography, breast sonography and computed tomography of the thorax, abdomen and pelvis. Aspirate and bone marrow biopsy was performed in all cases. Fluorodeoxyglucose-positron emission tomography and computed tomography (FDG-PET CT) was performed from 2005 for staging work-up.
Treatment response was assessed after initial treatment, and response criteria was determined following the guidelines published by the National Cancer Institute [13]. Complete remission (CR) was defined as the complete disappearance or no evidence of all detectable clinical and radiological evidence of disease and disease-related symptoms after systemic therapy or local therapy. Partial remission (PR) was defined as at least 50% decrease in the sum of the products of the greatest diameters (SPD). Stable disease (SD) was defined as less than a PR but is not progressive disease. Relapsed or progressive disease was considered when new lesions appeared or at least 50% increase from nadir in the SPD of any previously involved lesions after CR, or after PR or SD. Relapse-free survival (RFS) was defined as the time from diagnosis until disease relapse after CR or last follow-up, and disease-specific survival (DSS) was defined as the time from diagnosis until death as a result of the disease or last follow-up.
We used the Kaplan-Meier method to estimate the RFS and DFS. Statistical analyses were performed using PASW ver. 18.0 (IBM Co., Armonk, NY, USA).
A total of 9 patients were identified. Table 1 lists the baseline characteristics of patients. All patients were female. All specimen was diagnosed with DLBL (Fig. 1). All The median age at diagnosis was 47.9 years (range, 28 to 76 years). Eight patients initially visited the hospital with complaints of palpable breast mass without pain, and one patient presented with painful mass in the involved breast. No patients had B symptoms, including fever, night sweats or weight loss. No patients showed evidence of human immunodeficiency virus, previous organ transplantation or breast tumor. The median tumor size was 3.8 cm (range, 1.7 to 7.5 cm) in diameter. Five cases presented in the right breast and four cases in the left breast. Five patients were classified as IEA and four patients were classified as IIEA as based on the Ann Arbor staging system. Seven patients were classified as low risk group and the others were classified as low-intermediate risk group according to the IPI. Serum lactate dehydrogenase (LDH) was measured in all patients, and no patient had an elevated serum LDH.
The median follow-up time was 44 months (range, 18 to 90 months). First-line therapy and clinical outcomes are documented in Table 2. Five patients underwent surgery; one case of lumpectomy with sentinel lymph node biopsy because of uncertain diagnosis and four cases of excisional biopsy for diagnostic purposes. All patients received systemic chemotherapy with an anthracycline containing regimen (CHOP, cyclophosphamide, adriamycin, vincristine and prednisolone). Of those who received chemotherapy, five patients received rituximab-containing chemotherapy (R-CHOP) and one patient received bleomycine-containing chemotherapy (CHOP-Bleo). Four patients were treated with radiotherapy combined systemic chemotherapy at a total radiation therapy dose of 5,000 cGy in 25 fractions.
A CR was achieved in eight patients after first-line therapy. However, among the CR patients, three patients experienced disease relapse. One patient relapsed on the ipsilateral breast and two patients on the contralateral breast. Systemic relapse occurred in two patients, including one case in the maxillary sinus and one case in the bone marrow. The estimated mean relapse free period was 64 months (95% confidence interval [CI], 42 to 87 months). There was one case of SD (case no. 1); she expired 44 months after diagnosis because of an accident. In this study, there were two cases of disease-related death (cases no. 9 and 10), 73 months and 18 months after diagnosis, respectively. The estimated mean DSS was 77 months (96% CI, 61 to 93 months).
PBL is a rare disease entity, likely due to the paucity of lymphoid tissue in breast. By the dedicated efforts of several investigators, a number of studies have been undertaken regarding PBL, including a report of more than 200 patients from the International Extranodal Lymphoma Study Group [14] and a prospective study of 96 patients [15]. However, most reports regarding Korean PBL patients are retrospective and included small numbers of patients [16-19]. For these lesions, we report our experience of PBL in a single institution. To the best of our knowledge, this is one of the largest series in Korea women.
According to the definition set out by Wiseman and Liao [7], PBL is diagnosed when the breast is the first or major manifestation site of lymphoma without the involvement of lymphoma in any other organ, except the ipsilateral axillary lymph node. Patients with concurrent widespread disease or preceding extramammary lymphoma are also not defined as having PBL. Therefore, this definition encompasses only tumors classified as stage I or II disease according to the Ann Arbor staging system, and lymphomas involving the breast but not meeting these criteria are diagnosed as secondary breast lymphoma.
The most common clinical symptom in 60-100% of patients is a painless breast mass [9,20,21]. Other symptoms have been reported, including palpable lymph node, painful mass, local inflammation, or breast swelling. Similar to previous reports, all of our patients visited the hospital with the complaint of a palpable breast mass, with only one of them experiencing a localized pain. B symptoms are uncommon. The incidental mammographic detection rate has been previously noted to be about 12% [20]. Mammographic findings are nonspecific, and most lesions are oval-shaped and high-density lesions. There is no specific mammographic finding that could differentiate PBL from other invasive carcinomas of the breast. On ultrasound examination, lymphomas are detected as single, oval, and hypoechoic lesion without speculated margins or calcification [8,22,23].
Fine needle aspiration, core needle biopsy, and excisional biopsy are effective methods for evaluating breast mass and axillary lymph nodes. However, immunohistochemical or genetic studies are sometimes needed for exact diagnosis. In one case in our study (case no. 7), the pathologic department could not exactly diagnose lymphoma with the specimen from the core needle biopsy. Because the core needle biopsy was performed in outside institution, and the specimens were contaminated with the artifact. We performed a lumpectomy with sentinel lymph node biopsy based on the pre-operative diagnosis of "poorly differentiated invasive carcinoma". After the operation, immunohistochemical staining revealed that antibodies against the CD20, BCL-2 and BCL-6 were positive, and CD3 was negative on the surgically resected specimen. The specimen was diagnosed as DLBL of the breast based on microscopic morphological features and immunohistochemistry. Histologically, most PBLs are B-cell subtype. The majority of PLB cases in both our result and many published articles are DLBL, which account for 40-100% of PLB [20,24]. Low-grade subtypes of PBL, such as FL, MALT lymphoma, and marginal zone lymphoma are less commonly reported [8,25]. In our study, all cases were DLBL. In the pathologic laboratories, all specimens were showed no-particular arranged large lymphoid cells with vesicular and prominent nuclei. And the tissues were stained positively for the B-cell markers, including CD20 and Bcl-6, and negatively for the CD3, CD5 and CD10.
The prognosis of PBL has been reported that the behavior and clinical outcomes are similar to that of systemic lymphomas of the same histological types and stages. Therefore, prognostic factors for patient with PBL have been reported as histologic subtype, IPI, the use of anthracycline containing chemotherapy and/or radiation therapy [14,20], stage according to the Ann Arbor staging system [20], LDH level and age [9,26]. In a report by Ryan et al. [14] of 204 patients with primary DLBL of the breast, the median overall survival period was 8.0 years, and the median progression free period was 5.5 years. Our result showed similar results that estimated DSS and RFS were 6.4 years and 5.4 years, respectively. These results are improved over those of previous reports of Korean women with PBL. In a reports by Park et al. [18], the median overall survival and disease-free survival were calculated as 12 months and 6.5 months, respectively. We do not fully explain the exact cause of the differences in prognosis between our results and those of Park et al. [18], but the different prognosis may arise from differences in patients' characteristics and from improvements in treatment modalities.
There have been controversies regarding the treatment of PBL. In general, PBL treatment is similar to that of patients with systemic lymphoma of similar histologic type. Combination chemotherapy with or without radiation therapy is accepted as the main treatment method. A prospective study by Aviles et al. [15], found that the 10 year rate of event-free survival was 50%, 57%, and 83% for radiotherapy, chemotherapy, and combined therapy, respectively. Rituximab, a monoclonal antibody targeting the CD20 antigen, is known to have high efficacy for DLBL, and R-CHOP has been the mainstay for treatment of B-cell lymphoma [27]. However, due to the low incidence of PBL, there are no useful data regarding the efficacy of R-CHOP compared with CHOP for the treatment of PBL. In our study, R-CHOP was used for all patients diagnosed after July, 2005. All patients who received R-CHOP chemotherapy achieved CR, and among them, only one patient had a relapse in the contralateral breast. The use of CHOP and R-CHOP could be one of the reasons that the prognosis of our patients was better than the results of Park et al. [18]. Park et al. [18] which included patients diagnosed between 1989 and 2001. Three out of their patients were received anthracycline-containing chemotherapy, and no patients received a rituximab containing regimen.
Surgery is only recommended for diagnostic purposes, and is not recommended as a first line therapy, as extensive surgery may delay the beginning of chemotherapy [14]. Furthermore, Misra et al. [28] and Park et al. [18] suggested that surgery should be performed only after the local failure of systemic treatment. In our study, one patients received operation not for diagnostic purpose and the patient underwent a lumpectomy with sentinel lymph node biopsy because of an unsatisfactory diagnosis before the operation. Due to the small numbers in our series, no conclusions can be drawn regarding the prognosis according to stage and treatment methods.
In conclusion, due to the rarity of the PBL, there have been a few reports about PBL in Korean patients. In our study, all PBL cases are B-cell origin, with DLBL being the most common histologic type. A combined treatment modality has been known to have positive effects on prognosis, and surgery should be limited to diagnostic purposes. Further studies about PBL in Korean women are necessary to improve understanding of this disease. And, we also hope that further studies could reveal the impact of treatment protocol for Korean women with PBL.
Figures and Tables
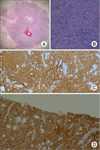 | Fig. 1Microscopic findings of primary diffuse large B-cell lymphoma (DLBL) of the breast (A: H&E, ×12.5). Note the large cells with no particular arrangement. Most cells are very large with vesicular and prominent nuclei, and have significant nuclear pleomorphism (B: H&E, ×200). The specimen shows immunoreactivity for CD20 (C, ×200) and BCL-2 (D, ×100). |
References
1. Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972. 29:252–260.
2. d'Amore F, Christensen BE, Brincker H, Pedersen NT, Thorling K, Hastrup J, et al. Clinicopathological features and prognostic factors in extranodal non-Hodgkin lymphomas. Danish LYFO Study Group. Eur J Cancer. 1991. 27:1201–1208.
3. Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noordijk EM. Primary extranodal non-Hodgkin's lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol. 2003. 14:131–139.
4. Ferguson DJ. Intraepithelial lymphocytes and macrophages in the normal breast. Virchows Arch A Pathol Anat Histopathol. 1985. 407:369–378.
5. Topalovski M, Crisan D, Mattson JC. Lymphoma of the breast: a clinicopathologic study of primary and secondary cases. Arch Pathol Lab Med. 1999. 123:1208–1218.
6. Gholam D, Bibeau F, El Weshi A, Bosq J, Ribrag V. Primary breast lymphoma. Leuk Lymphoma. 2003. 44:1173–1178.
7. Wiseman C, Liao KT. Primary lymphoma of the breast. Cancer. 1972. 29:1705–1712.
8. Domchek SM, Hecht JL, Fleming MD, Pinkus GS, Canellos GP. Lymphomas of the breast: primary and secondary involvement. Cancer. 2002. 94:6–13.
9. Ha CS, Dubey P, Goyal LK, Hess M, Cabanillas F, Cox JD. Localized primary non-Hodgkin lymphoma of the breast. Am J Clin Oncol. 1998. 21:376–380.
10. International Agency for Research on Cancer. Swerdlow S, Campo E, Harris NL, Jaffe ES, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon: World Health Organization;439.
11. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971. 31:1860–1861.
12. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993. 329:987–994.
13. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999. 17:1244.
14. Ryan G, Martinelli G, Kuper-Hommel M, Tsang R, Pruneri G, Yuen K, et al. Primary diffuse large B-cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol. 2008. 19:233–241.
15. Aviles A, Delgado S, Nambo MJ, Neri N, Murillo E, Cleto S. Primary breast lymphoma: results of a controlled clinical trial. Oncology. 2005. 69:256–260.
16. Jung TH, Chung KS, Kim WM, Yu BJ, Chung CH, Lee MJ, et al. A case of primary breast lymphoma. Korean J Hematol. 1992. 27:409–413.
17. Jung SW, Kim BS, Park JH, Kim SK, Seo HE, Shin DK, et al. A case of primary MALT lymphoma of the breast. Cancer Res Treat. 2001. 33:269–273.
18. Park YH, Kim SH, Choi SJ, Ryoo BY, Kang YK, Lee SS. Primary malignant lymphoma of the breast: clinicopathological study of nine cases. Leuk Lymphoma. 2004. 45:327–330.
19. Lim HJ, Cho KR, Kim I, Hwang KW, Seo BK, Woo OH, et al. Primary peripheral T-cell lymphoma of the breast: radiologic and pathologic findings. J Breast Cancer. 2010. 13:318–322.
20. Jeanneret-Sozzi W, Taghian A, Epelbaum R, Poortmans P, Zwahlen D, Amsler B, et al. Primary breast lymphoma: patient profile, outcome and prognostic factors. A multicentre Rare Cancer Network study. BMC Cancer. 2008. 8:86.
21. Brustein S, Filippa DA, Kimmel M, Lieberman PH, Rosen PP. Malignant lymphoma of the breast: a study of 53 patients. Ann Surg. 1987. 205:144–150.
22. Lyou CY, Yang SK, Choe DH, Lee BH, Kim KH. Mammographic and sonographic findings of primary breast lymphoma. Clin Imaging. 2007. 31:234–238.
23. Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology. 2007. 245:692–702.
24. Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999. 26:357–364.
25. Martinelli G, Ryan G, Seymour JF, Nassi L, Steffanoni S, Alietti A, et al. Primary follicular and marginal-zone lymphoma of the breast: clinical features, prognostic factors and outcome: a study by the International Extranodal Lymphoma Study Group. Ann Oncol. 2009. 20:1993–1999.
26. Hugh JC, Jackson FI, Hanson J, Poppema S. Primary breast lymphoma: an immunohistologic study of 20 new cases. Cancer. 1990. 66:2602–2611.
27. Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007. 26:3603–3613.
28. Misra A, Kapur BM, Rath GK. Primary breast lymphoma. J Surg Oncol. 1991. 47:265–270.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download