Abstract
Purpose
To evaluate shunt rate and discuss the resultsrelated to selective shunt placement during carotid endarterectomy (CEA) using routine awake test.
Methods
Patients with CEA from 2007 to 2011 were retrospectively reviewed from prospectively collected data. The need for shunt placement was determined by the awake test, based on the alteration in the neurologic examination. We collected data by using the clinical records and imaging studies, and investigated factors related to selective shunt such as collateral circulation and contralateral internal carotid artery (ICA) stenosis.
Results
There were 45 CEAs under regional anesthesia with the awake test in 44 patients. The mean age was 61.8 ± 7.1 years old. There were 82.2% (37/45) of males, and 68.9% (31/45) of symptomatic patients. Selective shunt placement had been performed in only two (4.4%) patients. Among them fewer cases (4%) had severe (stenosis >70%) contralateral ICA lesions, and more cases (91%) of complete morphology of the anterior or posterior circulation in the circle of Willis. There was no perioperative stroke, myocardial infarctionor death, and asymptomatic new brain lesions were detected in 4 patients (9%), including 2 cases of selective shunt placement.
Carotid endarterectomy (CEA) is an effective treatment modality to prevent strokes in patients with atherosclerotic carotid bifurcation disease [1]. There have been many debates regarding the need for and the adverse effects of shunt placement during CEA. The results of routine nonshunting are quite favorable: the stroke rate is approximately 2% [2]. However, this technique is not recommendable due to the legal problem. The stroke rate of routine shunting is 1.4% to 1.8% [3,4]. The possible adverse effects of shunting are intimal injury and embolism from atherosclerotic debris. However, some studies have shown that the benefits of shunting are superior to the risks against it [5]. In selective shunting, the results are good: the stroke rate is merely 1% [6]. During CEA with selective shunting, cerebral monitoring to assess ischemia from a cross-cramping of internal carotid artery (ICA) is necessary. The monitoring devices are diverse: stump pressure measurement [7], electroencephalogram [8], somatosensory evoked potentials [9], measurement of middle cerebral artery flow of transcranialdoppler [10], and cerebral oxygen saturation [11]. However, none of them can assess cerebral ischemia accurately for selective shunting. At present, the awake test under regional anesthesia is the most reliable method to predict selective shunting. The shunt rate is also lower than other monitoring modalities [10,12-15]. Many authors have reported the shunt rate to be 5% to 28% in selective shunting [10,12-18], and have suggested associated factors of shunt placement, including stenosis degree of ipsilateral and contralateral ICA, and completeness of the circle of Willis [17,18].
The aim of this study is to evaluate shunt rate and discuss the results related to selective shunt placement during CEA using routine awake test under regional anesthesia.
Patients who had undergone CEA from January 2007 to June 2011 were retrospectively reviewed from prospectively collected data. We collected data, including demographics, comorbidities, hemodynamic findings, morphological findings, surgical findings, morbidity, mortality, and survival from the medical records and imaging studies of the patients. If the patients had experienced a transient ischemic attack (TIA) or a stroke 6 months before surgery, they were considered symptomatic. We evaluated the shunt rate and identified the factors related to selective shunt placement during CEA using routine awake test under regional anesthesia.
All patients underwent brain magnetic resonance imaging (MRI), neck computed tomography angiography (CTA), carotid duplex scan, and transcranialdoppler preoperatively. A stenosis degree of the lesion was graded by the North American Symptomatic Carotid Endarterectomy Trial in CTA [1]. Completeness of the circle of Willis was evaluated from the morphological findings of CTA. Anterior circulation (ipsilateral A1 segment, anterior communicating artery, and contralateral A1 segment) and posterior circulation (posterior communicating artery and P1 segment) were classified as normal (diameter, ≥0.8 mm), hypoplastic (diameter, <0.8 mm), or absent according to the diameter measuring the component vessel segments [19].
We usually performed CEA under regional anesthesia with a deep cervical nerve block, while monitoring the neurologic symptoms of the patient. The need for a shunt placement was determined by the awake test, based on alterations in the neurologic examination, such as mental status deterioration and limb weakness that developed after at least 3 minutes of ICA clamping. All patients underwent a diffusion-weighted brain MRI evaluation to detect new brain lesions and neurologic examination by a neurologist the day after surgery. After that, a neurologic examination was performed by the neurologist every 3 months, and a CTA or carotid duplex scan was performed annually.
We had performed 48 CEAs from January 2007 to June 2011. Among them 45 CEAs under regional anesthesia with the awake test in 44 patients were included in this study and 3 CEAs under general anesthesia were excluded. The mean follow-up duration was 21.4 ± 14.6 months (range, 0 to 53 months). The mean age was 61.8 ± 7.1 years old (range, 47 to 78 years). There were 82.2% (37/45) of males. There were 68.9% (31/45) of symptomatic patients and 31.1% (14/45) of asymptomatic patients. Among symptomatic patients, there were 61.3% (19/31) cases of strokes and 38.7% (12/31) cases of TIA history. The mean time interval to surgery after last symptomatic attack was 56.1 ± 33.5 days (range, 9 to 134 days). Baseline characteristics and comorbidities are shown in Table 1.
Selective shunt placement was undertaken in only two patients (4.4%). One was inserted before endarterectomy during a conventional technique, and the other was performed after endarterectomy during an eversion technique. Eversion technique was performed in 76% (34/45) of all cases. There was no conversion from eversion technique to conventional technique (Table 2).
Hemodynamic and morphologic findings of ICA are shown in Table 3. In ipsilateral ICA lesions, severe stenosis (>70%) was 93.3%. In contralateral ICA lesions, there was no occlusion. Severe stenosis (>70%) and moderate stenosis (50% to 69%) were 4.4%, and mild stenosis (<50%) was 91.1% (Fig. 1).
Complete morphology of the anterior circulation was 60.0% (27/45), as well as the posterior circulation (Table 4). Complete morphology of the anterior or posterior circulation was considered good collateral circulation (91.1%, 41/45). Hypoplastic or absent category was considered poor collateral circulation (8.9%, 4/45). Almost twenty-nine percent (28.9%, 13/45) had an entirely complete circle of Willis (Fig. 2).
Two patients who have selective shunt placement do not have any factors relating to shunt placement.
There was no perioperative stroke. However, new asymptomatic brain lesions were detected in 4 patients (8.9%), including 2 cases of selective shunt placement; 2 cases of eversion technique, and 2 cases of conventional patch technique. All lesions were on the ipsilateral side. Other morbidities are shown in Table 5. There was no perioperative death.
There was no myocardial infarction, death, recurring stroke, or restenosis on imaging study among the patients during a follow-up time of 21.4 ± 14.6 months after discharge, except for one case of loss to follow-up.
Many authors have suggested the factors associated with selective shunt placement. These are stenosis degree of ipsilateral and contralateral ICA, and completeness of the circle of Willis. Tan et al. [17] have reported that stenosis degree of ipsilateral ICA is relevant to selective shunt placement. The patients with moderate stenosis (50% to 79%) are more likely to be associated with electroencephalography changes during clamping compared to those with severe stenosis (80% to 99%). They believe that this may be due to the greater relative loss of ipsilateral flow during clamping in the moderate stenosis group [17].
We can evaluate the collateral circulation from contralateral ICA and the circle of Willis. Some studies have reported that stenosis degree of contralateral ICA is relevant to selective shunt placement [13,17,20]. The occlusive lesions have a higher shunt rate, 19% to 55% [13,20]. In these studies, the patients with occlusive lesions are 10% to 13%.
Lee et al. [18] have reported that incompleteness of the circle of Willis is relevant to intraoperative cerebral ischemia during ICA clamping in patients with contralateral ICA occlusion. In patients with no occlusion of contralateral ICA, there is no ischemia regardless of the incompleteness of the circle of Willis. In this study, good collateral circulation of the circle of Willis is 76.1%, and contralateral ICA occlusion is 18% in which cerebral ischemia occurs 57% [18]. Hendrikse et al. [21] have reported that in patients with unilateral ICA stenosis, vessel diameters of the circle of Willis are larger than in control subjects [21].
Based on the above studies, a relatively lower shunt rate of our institution is explainable. In our study, selective shunt placement under regional anesthesia with an awake test is 4.4% (2/45), which is lower than 5% to 28% reported in many other studies [10,12-18]. The low shunt rate may be due to the following reasons. In our study, ipsilateral ICA lesions show fewer cases (6.7%) of moderate stenosis (50% to 69%) and more cases (93.3%) of severe stenosis (>70%). In contralateral ICA lesions, there are no occlusion, and fewer cases (4.4%) of severe stenosis (>70%), and more cases (91.1%) of mild stenosis (<50%). There are more cases (91.1%) of good collateral circulation of the circle of Willis. However, the shunt rate prevalence in our institution cannot be accurately compared to other studies, because most authors do not describe precisely the proportion of patients with the related factors described above [10,12-18]. On the other hand, patients who have selective shunt placement have no related factors mentioned above, where there are no explainable reasons.
The followings are not known to be related factors: presence of ipsilateral preoperative symptoms, hypertension, coronary artery disease, diabetes, age, gender, smoking, preemptive intraoperative blood pressure manipulation to 20% or more above baseline before cross-clamping [17], and cerebral oxygen saturation [11].
The present study shows that the treatment outcomes of the CEA with a routine awake test are satisfactory. In our study, the stroke rate is 0%; others have reported 0% to 4.0% [6,22-25]. Our finding shows new brain lesions occurring 8.9% (4/45). Others have reported 10% to 17% [26-28]. These lesions are related to thromboembolic phenomena during carotid surgery, and selective shunt may reduce shunt rate and new brain lesions [29]. Schnaudigel et al. [26] have reported that selective shunting reduces new brain lesions from 16% to 6%, significantly more than routine shunting. There is no myocardial infarction; others have reported 0.2% to 1.4% [5,22,23]. Nerve injury occurs 2.2% (1/45); others have reported 3.6% [5,25]. Hyperperfusion syndrome occurs 2.2% (1/45); others have reported 1% to 3% [30]. There are no deaths related to surgery; others have reported 0% to 1.5% [22,24,25]. There is no resteonosis during the follow-up period; others have reported 0.7% [5].
At the same time, these treatment outcomes of CEA in our institution cannot be accurately compared with other studies described above because the baseline characteristics are different. In our study, 68.9% are symptomatic patients; some studies have only symptomatic patients [5,27], while others have fewer (30% to 68%) [22-24,28]. In our study, the mean age is 61.8, but in other studies, the mean age was approximately 70 [5,22-24,27,28]. However, despite these limitations, it is obvious that the treatment outcomes of our study are satisfactory.
Our studies are limited by a small number of patients. As there are only 2 cases of selective shunt placement, the analysis of associated factors is impossible. There is also less power in the relationship between shunt placement and complications such as new brain lesions. Thus, future research should consider using a larger number of patients.
In conclusion, the present study shows that using a routine awake test during CEA under regional anesthesia has a lower shunt rate (4.4%). The reasons for relatively lower shunt rate in this study may be that our study had included more cases of good collateral circulation to brain: fewer cases (4%) of contralateral ICA lesions, and more cases (91%) of complete morphology of the anterior or posterior circulation in the circle of Willis. It is better not to shunt if possible, because shunt placement can cause adverse events. Therefore CEA under routine awake test could be safe and feasible method with low shunt placement rate in selected patients
Figures and Tables
 | Fig. 1Categories of contralateral internal carotid artery (ICA) stenosis are shown in the computed tomography angiography. Severe stenosis (>70%) was in the ipsilateral ICA lesions (arrow of A, B and C). Severe stenosis (>70%) (arrowhead of A), moderate stenosis (50% to 69%) (arrowhead of B) and mild stenosis (<50%) (arrowhead of C) were in the contralateral ICA lesions. |
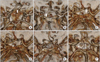 | Fig. 2Morphologic findings of the circle of Willis in the 3-dimentional reconstruction of computed tomography angiography show normal (diameter, ≥0.8 mm) (arrow of A), hypoplastic (diameter, <0.8 mm) (arrow of B) and absent (arrow of C) anterior circulation and normal (arrowhead of D), hypoplastic (arrowhead of E) and absent (arrowhead of F) posterior circulation. |
References
1. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991. 325:445–453.
2. Frawley JE, Hicks RG, Gray LJ, Niesche JW. Carotid endarterectomy without a shunt for symptomatic lesions associated with contralateral severe stenosis or occlusion. J Vasc Surg. 1996. 23:421–427.
3. Hertzer NR, O'Hara PJ, Mascha EJ, Krajewski LP, Sullivan TM, Beven EG. Early outcome assessment for 2228 consecutive carotid endarterectomy procedures: the Cleveland Clinic experience from 1989 to 1995. J Vasc Surg. 1997. 26:1–10.
4. Lee KB, Lee KH, Chung CS, Kim GM, Byun HS, Jeon P, et al. Carotid endarterectomy: analysis of early complications (<30 days) and risk factors for postoperative new brain infarction. J Korean Surg Soc. 2009. 77:195–201.
5. Gumerlock MK, Neuwelt EA. Carotid endarterectomy: to shunt or not to shunt. Stroke. 1988. 19:1485–1490.
6. Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981. 56:533–543.
7. Calligaro KD, Dougherty MJ. Correlation of carotid artery stump pressure and neurologic changes during 474 carotid endarterectomies performed in awake patients. J Vasc Surg. 2005. 42:684–689.
8. Hans SS, Jareunpoon O. Prospective evaluation of electroencephalography, carotid artery stump pressure, and neurologic changes during 314 consecutive carotid endarterectomies performed in awake patients. J Vasc Surg. 2007. 45:511–515.
9. Wober C, Zeitlhofer J, Asenbaum S, Claeys L, Czerny M, Wolfl G, et al. Monitoring of median nerve somatosensory evoked potentials in carotid surgery. J Clin Neurophysiol. 1998. 15:429–438.
10. Belardi P, Lucertini G, Ermirio D. Stump pressure and transcranial Doppler for predicting shunting in carotid endarterectomy. Eur J Vasc Endovasc Surg. 2003. 25:164–167.
11. Friedell ML, Clark JM, Graham DA, Isley MR, Zhang XF. Cerebral oximetry does not correlate with electroencephalography and somatosensory evoked potentials in determining the need for shunting during carotid endarterectomy. J Vasc Surg. 2008. 48:601–606.
12. Illig KA, Sternbach Y, Zhang R, Burchfiel J, Shortell CK, Rhodes JM, et al. EEG changes during awake carotid endarterectomy. Ann Vasc Surg. 2002. 16:6–11.
13. Lawrence PF, Alves JC, Jicha D, Bhirangi K, Dobrin PB. Incidence, timing, and causes of cerebral ischemia during carotid endarterectomy with regional anesthesia. J Vasc Surg. 1998. 27:329–334.
14. McCarthy RJ, Walker R, McAteer P, Budd JS, Horrocks M. Patient and hospital benefits of local anaesthesia for carotid endarterectomy. Eur J Vasc Endovasc Surg. 2001. 22:13–18.
15. McCleary AJ, Maritati G, Gough MJ. Carotid endarterectomy; local or general anaesthesia? Eur J Vasc Endovasc Surg. 2001. 22:1–12.
16. Aburahma AF, Stone PA, Hass SM, Dean LS, Habib J, Keiffer T, et al. Prospective randomized trial of routine versus selective shunting in carotid endarterectomy based on stump pressure. J Vasc Surg. 2010. 51:1133–1138.
17. Tan TW, Garcia-Toca M, Marcaccio EJ Jr, Carney WI Jr, Machan JT, Slaiby JM. Predictors of shunt during carotid endarterectomy with routine electroencephalography monitoring. J Vasc Surg. 2009. 49:1374–1378.
18. Lee JH, Choi CG, Kim DK, Kim GE, Lee HK, Suh DC. Relationship between circle of Willis morphology on 3D time-of-flight MR angiograms and transient ischemia during vascular clamping of the internal carotid artery during carotid endarterectomy. AJNR Am J Neuroradiol. 2004. 25:558–564.
19. Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. 1999. 30:2671–2678.
20. Schneider JR, Droste JS, Schindler N, Golan JF, Bernstein LP, Rosenberg RS. Carotid endarterectomy with routine electroencephalography and selective shunting: Influence of contralateral internal carotid artery occlusion and utility in prevention of perioperative strokes. J Vasc Surg. 2002. 35:1114–1122.
21. Hendrikse J, Rutgers DR, Klijn CJ, Eikelboom BC, van der Grond J. Effect of carotid endarterectomy on primary collateral blood flow in patients with severe carotid artery lesions. Stroke. 2003. 34:1650–1654.
22. Sternbach Y, Illig KA, Zhang R, Shortell CK, Rhodes JM, Davies MG, et al. Hemodynamic benefits of regional anesthesia for carotid endarterectomy. J Vasc Surg. 2002. 35:333–339.
23. Aleksic M, Luebke T, Brunkwall J. Outcome of carotid endarterectomy under local anaesthesia with respect to the patients' risk profile. Vasa. 2009. 38:225–233.
24. Palombo D, Lucertini G, Mambrini S, Zettin M. Subtle cerebral damage after shunting vs non shunting during carotid endarterectomy. Eur J Vasc Endovasc Surg. 2007. 34:546–551.
25. Rerkasem K, Rothwell PM. Routine or selective carotid artery shunting for carotid endarterectomy and different methods of monitoring in selective shunting. Stroke. 2010. 41:e53–e54.
26. Schnaudigel S, Groschel K, Pilgram SM, Kastrup A. New brain lesions after carotid stenting versus carotid endarterectomy: a systematic review of the literature. Stroke. 2008. 39:1911–1919.
27. Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol. 2010. 9:353–362.
28. Lacroix V, Hammer F, Astarci P, Duprez T, Grandin C, Cosnard G, et al. Ischemic cerebral lesions after carotid surgery and carotid stenting. Eur J Vasc Endovasc Surg. 2007. 33:430–435.
29. de Borst GJ, Moll FL, van de Pavoordt HD, Mauser HW, Kelder JC, Ackerstaf RG. Stroke from carotid endarterectomy: when and how to reduce perioperative stroke rate? Eur J Vasc Endovasc Surg. 2001. 21:484–489.
30. Bouri S, Thapar A, Shalhoub J, Jayasooriya G, Fernando A, Franklin IJ, et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg. 2011. 41:229–237.




 ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download