Abstract
Purpose
Few studies have examined whether bioengineering can improve fecal incontinence. This study designed to determine whether injection of porous polycaprolactone beads containing autologous myoblasts improves sphincter function in a dog model of fecal incontinence.
Methods
The anal sphincter of dogs was injured and the dogs were observed without and with (n = 5) the injection of porous polycaprolactone beads containing autologous myoblasts into the site of injury. Autologous myoblasts purified from the gastrocnemius muscles were transferred to the beads. Compound muscle action potentials (CMAP) of the pudendal nerve, anal sphincter pressure, and histopathology were determined 3 months after treatment.
Results
The amplitudes of the CMAP in the injured sphincter were significantly lower than those measured before injury (1.22 mV vs. 3.00 mV, P = 0.04). The amplitudes were not different between dogs with and without the injection of autologous myoblast beads (P = 0.49). Resting and squeezing pressures were higher in dogs treated with autologous myoblast beads (2.00 mmHg vs. 1.80 mmHg; 6.13 mmHg vs. 4.02 mmHg), although these differences were not significant in analyses of covariance adjusted for baseline values. The injection site was stained for smooth muscle actin, but showed evidence of foreign body inflammatory reactions.
Conclusion
This was the first study to examine whether bioengineering could improve fecal incontinence. Although the results did not show definite evidence that injection of autologous myoblast beads improves sphincter function, we found that the dog model was suitable and reliable for studying the effects of a potential treatment modality for fecal incontinence.
Fecal incontinence is a socially disabling disorder that profoundly impacts on all aspects of quality of life. It is thought to effect 2% to 15% of community-dwelling adults [1,2]. Fecal incontinence is considered to be an aging disease because its prevalence increases to 13% among people older than 50 years [3], and is an iatrogenic disease that develops in 60% of rectal cancer patients after intersphincteric resection [4]. However, the treatment of fecal incontinence is an area of uncertainty because data from randomized trials are lacking to establish optimal approaches for its treatment [5]. Although injection of a bulking agent has been considered as a minimally invasive treatment [6], its efficacy is limited by several factors, including reabsorption, migration [7], fat embolism, and granuloma formation [8].
Approaches aimed at regenerating functional muscle have been proposed as a therapeutic tool for fecal incontinence [9]. Some pioneers in the field of coloproctology have shown that autologous adipose stem cells may be used to treat fistulas in patients with Crohn's disease; these cells did not migrate or cause embolism [10]. Several reports showed that injection of cells into the anal sphincter improved functional outcomes in rat [11-14] and rabbit [15] models of fecal incontinence. Additionally, several clinical trials have tested whether the injection of myoblasts improves incontinence, but there was no histologic evidence demonstrating the induction of functional muscle [16]. We consider that cell therapy alone without a bioscaffold produced by bioengineering technique would provide limited functional and structural improvements in fecal incontinence patients with large structural deformities [17], which differs from urinary incontinence, which shows less marked structural deformities.
Until recently, no studies have used bioengineering techniques to improve fecal incontinence. In our previous study, the use of polycarprolactone porous beads [18] facilitated smooth muscle regeneration and improved electrical contractility in vitro evaluation [19], which indicates that these beads may be useful as an injectable bioactive bulking agent for treating incontinence [20]. Therefore, the present study was designed to determine whether the injection of porous polycaprolactone beads containing autologous myoblasts could improve sphincter function in a dog model of fecal incontinence.
This protocol was reviewed and approved by the Animal Care and Use Committee of Seoul National University Bundang Hospital (Protocol No. 64-2010-037). Normal 10-week-old female mongrel dogs (19 to 22 kg) were used in these experiments. The animals were intubated and mechanically ventilated with a Zoletil (Virbac, Carros, France) + Rompun (Bayer Korea, Seoul, Korea) mixture to maintain surgical anesthesia. All procedures were performed in the lithotomy position. Ketoprofen was used for pain control and cefazolin was injected intravenously before and immediately after surgery. In each animal, sphincter injury was induced by partial extraction of approximately 25% of the posterior internal/external anal sphincter using electrocautery.
After inducing anal sphincter injury, the dogs were randomly allocated to two groups and received without (n = 5) or with (n = 5) an injection of porous polycaprolactone beads containing autologous myoblasts into the injured sphincter. In the autologous myoblast bead injected group, a muscle biopsy of 1 cm3 in size was taken from gastrocnemius muscle to isolate myoblasts at the time of the surgery to injure the anal sphincter. Three weeks later, the porous polycaprolactone beads containing the autologous myoblasts were injected into the site of injury in the healing process.
Compound muscle action potentials (CMAP) were measured and manometry of the anal sphincter was performed just before injury and 3 months after the intervention. The anal sphincter was removed for histopathological evaluation.
We stimulated the inferior rectal branch of the pudendal nerve located 3 cm proximal to the anal verge using a stimulator constructed in-house with a needle evoked potential electrode (Oxford Instrument Medical Systems, Oxfordshire, UK). CMAPs were recorded from active and reference electrodes using a Teflon-coated monopolar needle electrode (37 mm long, 0.30 mm diameter, Oxford Instrument Medical Systems) inserted into the superficial external anal sphincter muscles. A portable two-channel electromyography/nerve conduction velocity system (Medelec Synergy, Oxford Instrument Medical Systems) was used with a filtering frequency of 10 Hz to 10 kHz, sweep speed of 1 ms/division and sensitivity of 1 mV/division. The mean amplitude and area of the CMAP were measured for five pudendal nerve conduction studies in each dog.
Anal sphincter pressure was measured by real-time pressure monitoring (Creta Co., Seoul, Korea) using a pressure balloon sensor (unit, mmHg; sensitivity 0.06 kPa, sensitivity 5 mV, threshold 1.5 V; Boston Scientific, Place Natick, MA, USA) introduced into the animal rectum. The sensor probe was inserted about 1.5 cm into the anal orifice to be placed in the middle of the anal sphincter muscle. The baseline resting pressure was determined as the mean pressure in the noncontractile state after balloon insertion. The squeezing pressure was defined as the maximal pressure generated by the sphincter during tetanic stimulation of the pudendal nerve (50 Hz, 50 mA stimulus intensity, 5 seconds) and was measured at least three times in each dog.
Myoblasts were purified by the modified preplate technique, as previously described [21,22]. To characterize the cultured cells, the cells were cultured on chamber slides and fixed in cold methanol for 10 minutes. The cells were then incubated for 1 hour with rabbit polyclonal anti-Pax7 primary antibody (1:300; Lifespan Bioscience, Seattle, WA, USA), followed by labeling with polymer-horseradish peroxidase (HRP) antimouse secondary antibody (EnVision + System-HRP [3.3'-diaminobenzidine {DAB}], Dako, Glostrup, Denmark). Some cells were stained without the primary antibody as a negative control. Nuclei were stained with 3.3'-diaminobenzidine chromogen solution (Dako).
For the fusion assay, the purified cells were resuspended in differentiation medium (Dulbecco's modified eagle medium/F12 (1:1) + 2% horse serum + 1% penicillin/streptomycin). Myoblast differentiation was examined immunocytochemically using myosin heavy chain (MHC) monoclonal antibody (1:25; Novus Biologicals, Littleton, CO, USA) and α-smooth muscle actin (SMA) monoclonal antibody (prediluted, Dako). The cells were visualized under a microscope by a pathologist blinded to the study design.
Polycaprolactone/Pluronic F127 porous beads (size range, 200 to 300 µm) were prepared as previously described [18-20]. Briefly, polycaprolactone (Mw 43,000-50,000; Polysciences, Warrington, PA, USA) and Pluronic F127 (EG99PG65EG99, Mw 12,500; BASF, Parsippany, NJ, USA) were used to fabricate the porous polycaprolactone beads. The beads were first fabricated by isolated particle melting to generate nonporous beads, followed by melt-molding particulate-leaching to generate porous beads, as described elsewhere [18]. Ultrapure grade water (>18 mΩ) was purified using a Milli-Q purification system (Millipore Co., Billerica, MA, USA). For animal studies, the porous beads were sterilized by ethylene oxide, and the Pluronic F127 solution was autoclaved before use.
As previously described [19], to seed the cells into the porous beads, the beads were immersed in a needle tip-stopped syringe filled with 2 mL of the cell suspension (cell density, 3 × 107 cells/mL). The cell suspension was transferred into the porous beads (10 mg) under negative pressure when the syringe piston was pulled out. The beads were maintained for 2 hours at 37℃ in an incubator with a humidified 5% CO2 atmosphere for the cells to adhere to the beads. To ensure even delivery of the cell-seeded porous beads during injection, the beads were uniformly dispersed in Pluronic F127 solution (in phosphate buffered saline, 25 wt%; gelation temperature, about 17℃) with a bead:solution ratio of 1:12 (w/v). The cell-seeded porous beads were injected into the injured tissue using a syringe fitted with a 19 G needle. The beads in the gelled state of Pluronic F127 at room temperature could be homogeneously injected through the syringe needle without blocking the needle.
At the end of each experiment, the dogs were euthanized by an intravenous overdose of KCl after intramuscular injection of Zoletil/Rompun (4.4 + 2.2 mg/kg). After the dogs were euthanized, the rectum and anal canal were dissected, immersion fixed in neutrally buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E).
After H&E slide review, representative blocks were selected. Four-micrometer sections were cut from the original blocks and stained for MHC mouse monoclonal antibody (1:200; Novus Biologicals) and SMA mouse monoclonal antibody (prediluted, Dako). The slides were deparaffinized, and hydrated in graded ethanol. Antigen retrieval was performed by incubating tissue sections in a microwave oven in 0.01 M citrate buffer (pH 6.0) for 15 minutes. The slides were incubated with primary antibody, followed by labeling with polymer-HRP antimouse secondary antibody (EnVision + System-HRP [DAB], Dako). For detection, 3.3'-diaminobenzidine chromogen solution (Dako) were applied. Normal skeletal and smooth muscle cells were used as internal positive controls, and fibroblasts and epithelial cells were used as internal negative controls. Some slides were stained without the primary antibody as a negative control.
Data are expressed as the median and interquartile range. Paired t-tests and Wilcoxon signed rank test were used for comparisons of functional outcomes measured before sphincter injury and at 3 months after injury in the dogs without the injection of porous polycaprolactone beads containing autologous myoblasts. Functional outcomes were compared by analysis of covariance (ANCOVA), with the corresponding baseline measurement as the covariate. All statistical tests were two-sided and P < 0.05 was considered significant. All analyses were performed using the IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA).
All animals recovered uneventfully from surgery, and all surgical incisions healed without complications.
We isolated myoblasts from gastrocnemius muscles, and the cells were characterized by immunocytochemical staining. The cultured myoblasts were spindle shaped, and typical myoblast characteristics, as determined by Pax7 staining (Fig. 1). These cells, with potential differentiation capability based on MHC and SMA staining, were used in subsequent experiments.
Three months after injuring the anal sphincter, the median amplitude of the CMAPs were significantly decreased compared with that measured before sphincter injury (1.22 mV vs. 3.00 mV, P = 0.04) (Table 1). Representative traces of CMAP are shown in Fig. 2. In the untreated sphincter injury group, there were no differences in the area of the CMAP between 3 months after sphincter injury and before injury (2.84 mV vs. 3.45 mV, P = 0.69). Meanwhile, in the autologous myoblast bead-treated group, the amplitude and area of the CMAP were not significantly different to those in the untreated group based on ANCOVA adjusted for baseline values.
Three months after injuring the anal sphincter, both resting pressure and squeezing pressure were significantly decreased compared with the values measured before injury (respectively, 1.80 mmHg vs. 3.50 mmHg, P = 0.04; 4.02 mmHg vs. 9.00 mmHg, P = 0.04) (Table 1). Representative traces of anal pressure are shown in Fig. 3. Resting pressure and squeezing pressure were higher in the autologous myoblast bead-treated group compared with untreated injured group, although not significantly based on ANCOVA adjusted for baseline values.
The external anal sphincter is composed of striated muscle while the internal anal sphincter shows characteristics of smooth muscle (Fig. 4A). The uninjured normal anal sphincter composed of smooth and skeletal muscle stained positively for both MHC and SMA (Fig. 4B, C). The injured anal sphincter showed damage to the muscle fibers with intermysial fibrosis and focal interstitial inflammatory cell infiltration (Fig. 4D). In addition, immunostaining showed atrophied striated and smooth muscle and loss of them (Fig. 4E, F). The anal sphincter of dogs treated with the autologous myoblast beads had a hypercellular inflammatory infiltrate that was partially surrounded by fibrous connective tissue (Fig. 4G), but the tissue showed no MHC staining and weak SMA staining (Fig. 4H, I). This inflammatory infiltrate was heterogeneous, consisting mainly of multinucleated giant cells containing foreign material in their cytoplasm, foamy macrophages and some lymphocytes; these findings are consistent with a foreign body reaction.
To our knowledge, this is the first study to examine whether a bioengineering technique could improve fecal incontinence. Unfortunately, the results did not support the use of porous polycaprolactone beads containing autologous myoblasts as a tool to improve sphincter function in a dog model of fecal incontinence. This is because the injected myoblasts did not integrate within the injured sphincter or play a functional role within the bioscaffold, even though we used porous beads that were previously found to stimulate the smooth muscle cell differentiation [19].
We consider that bioengineering techniques are necessary for patients with incontinence associated with large structural deformities. To date, several types of cells have been proposed for the regeneration of the anal sphincter muscle, including muscle-derived stem cells [12], bone marrow-derived mesenchymal stem cells [11], muscle progenitor cells [15], and myoblasts [23]. In the present study, we found limited differentiation of the myoblasts on histologic analysis, while a marked foreign body reaction was apparent around the site of injury. From these results, we consider that the pore size of the beads (25 to 50 µm) is too small to allow the entry of myoblasts, preventing integration of the cells and beads, even though the porous polycaprolactone beads containing autologous myoblasts are immune-compatible, nonmigratory, durable, and efficacious in other settings. The unexpected outcomes may also be related to an adverse immune response or granuloma formation following injection of an agent [8,24], but no foreign body reactions were reported in a Cochrane review [25]. In this study, the beads (200 to 300 µm in diameter) were larger than the maximum size of particles that can be ingested by macrophages. Clearly, the physiologic effects of foreign body reaction following injections of an agent, similar to that observed here, should be examined in animal models because no clinical studies have investigated the distribution of injected cells, or whether they remain viable. Another issue that needs to be evaluated in the context of injections of porous beads is whether the injected cells can differentiate into myofibers with contractile ability and can undergo reinnervation for functional muscle regeneration [12]. No studies have focused on tissue reinnervation in the field of muscle engineering, although some animal studies have indicated that muscle reinnervation may be possible by electrical stimulation, or the addition of Schwann cells [26,27].
In this study, the dog model was suitable and reliable for studying the effects of a potential treatment modality for fecal incontinence that could provide a greater step towards clinical trials than would studies in rats [11-14] or rabbits [15]. Indeed, a large animal model is needed to examine the specific effects on the anal sphincter, as the anal sphincter in rats is only a few millimeters thick and is almost impossible to isolate in vivo [28]. In this study using dogs, we were able to perform functional studies, including measurement of CMAPs of pudendal nerve. Thus, the dog model offers several advantages over rat and rabbit models, although it is limited by cost and convenience, which means rat models are often preferred over larger animals for studying novel treatments. Some functional studies in rats measuring in vitro contractility [11,12], and electromyography or manometry in vivo [13-15] have been reported quite recently. To our knowledge, ours is the first in vivo functional study using manometry and measurement of CMAPs in dogs. CMAP is considered to be a functional indicator of muscle function, although the amplitude of CMAPs is related to the diameter of the closest muscle fibers and the density of the muscle fibers comprising the motor unit closest to the electrode [29,30]. Therefore, the amplitude of CMAPs could be used as a marker of fecal incontinence in dogs.
In adult vertebrates, myogenesis is mainly sustained by a subpopulation of myogenic cells known as satellite cells, which are activated into myoblasts in response to stimuli, particularly tissue injury. Cultured myoblasts can also be integrated into the host muscle. The main advantages of autologous transplantation are the avoidance of tissue rejection, and the absence of ethical concerns, unlike stem cell therapy. Therefore, we used autologous myoblasts for the regeneration of the anal sphincter, as in earlier studies [16]. Further studies are needed to confirm whether the injection of porous polycaprolactone beads containing autologous myoblasts can stimulate muscle regeneration.
Our study has some limitations to discuss. First, the absence of statistical significance could be due to type 2 error, as there were only five dogs in each group, although some trends were evident. Second, we did not label the cells to enable cell tracking, a limitation of many studies of cell therapy. Further studies are planned to evaluate cell survival, distribution, differentiation, integration, and functional contributions of the cells using appropriate tracking methods. Third, CMAPs or anal manometry in the present study is not used as a stratifying tool for the severity of fecal incontinence in human, and was based on practical constraints of animal study that is not reliable with subjective tool of gas, liquid or solid stool incontinence.
Until recently, no definitive bioengineering approaches have been developed for fecal incontinence, as effective treatments require a reliable cell source, tissue vascularization, tissue function enhancement, biomaterial development, and tissue innervation. Although this study did not confirm that the injection of porous polycaprolactone beads containing autologous myoblasts can be used as a tool to improve sphincter function, we found that the dog model was suitable and reliable for studying the effects of a potential treatment modality for fecal incontinence.
Figures and Tables
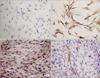 | Fig. 1Monolayer culture of dog skeletal muscle cells. (A) Negative control for Pax7. (B) Immunocytochemical staining for Pax7. (C) Immunocytochemical staining for myosin heavy chain after cell fusion. (D) Immunocytochemical staining for smooth muscle actin after cell fusion (A-D, ×400). |
 | Fig. 2Representative compound muscle action potential traces. (A) Normal anal sphincter before injury. (B) Three months after anal sphincter injury. (C) Three months after injection of porous polycaprolactone beads containing autologous myoblasts. The compound muscle action potentials were recorded after electrical stimulation of the pudendal nerve. |
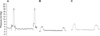 | Fig. 3Representative anal sphincter pressure traces. (A) Normal anal sphincter before injury. (B) Three months after anal sphincter injury. (C) Three months after injection of porous polycaprolactone beads containing autologous myoblasts. The squeezing pressure was recorded after electrical stimulation from the resting pressure. |
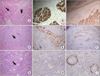 | Fig. 4Hematoxylin/eosin staining and immunostaining of the anal sphincter. (A-C) Normal anal sphincter. Normal outer striated muscle fibers (arrow) and internal smooth muscle layer (arrowhead) are indicated. (D-F) Three months after anal sphincter injury. Immunostaining showed extensive damage to the muscle fibers with cytoplasmic fibrosis and focal interstitial inflammatory cell infiltration (arrow), and atrophy of the muscle fibers. (G-I) Three months after injection of porous polycaprolactone beads containing autologous myoblasts. There was a marked foreign body reaction characterized by the presence of numerous giant cells and foamy macrophages (arrow), with weak staining for α-smooth muscle actin (A, D, G: hematoxylin-eosin staining; B, E, H: immunostaining of myosin heavy-chain; C, F, I: immunostaining of α-smooth muscle actin; A-G, ×100; H and I, ×200). |
ACKNOWLEDGEMENTS
This work was supported by a grant from the Seoul National University Bundang Hospital (Grant No. 04-2009-001), and the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (Grant No. 2012-0001052). The authors thank Dr. Young-Hoon Kim, Dr. So-Yeon Kim and, M.S. Ji-Yeon Hwang for their assistance and advice.
References
1. Nelson R, Norton N, Cautley E, Furner S. Community-based prevalence of anal incontinence. JAMA. 1995. 274:559–561.
2. Macmillan AK, Merrie AE, Marshall RJ, Parry BR. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum. 2004. 47:1341–1349.
3. Roberts RO, Jacobsen SJ, Reilly WT, Pemberton JH, Lieber MM, Talley NJ. Prevalence of combined fecal and urinary incontinence: a community-based study. J Am Geriatr Soc. 1999. 47:837–841.
4. Ito M, Saito N, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis Colon Rectum. 2009. 52:64–70.
5. Wald A. Clinical practice: fecal incontinence in adults. N Engl J Med. 2007. 356:1648–1655.
6. Shafik A. Polytetrafluoroethylene injection for the treatment of partial fecal incontinence. Int Surg. 1993. 78:159–161.
7. Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001. 166:1350–1353.
8. Malizia AA Jr, Reiman HM, Myers RP, Sande JR, Barham SS, Benson RC Jr, et al. Migration and granulomatous reaction after periurethral injection of polytef (Teflon). JAMA. 1984. 251:3277–3281.
9. Williams NS. Pelvic floor disorders and reconstruction: what next? Dis Colon Rectum. 2008. 51:1309–1311.
10. Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005. 48:1416–1423.
11. Lorenzi B, Pessina F, Lorenzoni P, Urbani S, Vernillo R, Sgaragli G, et al. Treatment of experimental injury of anal sphincters with primary surgical repair and injection of bone marrow-derived mesenchymal stem cells. Dis Colon Rectum. 2008. 51:411–420.
12. Kang SB, Lee HN, Lee JY, Park JS, Lee HS, Lee JY. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum. 2008. 51:1367–1373.
13. Salcedo L, Damaser M, Butler R, Jiang HH, Hull T, Zutshi M. Long-term effects on pressure and electromyography in a rat model of anal sphincter injury. Dis Colon Rectum. 2010. 53:1209–1217.
14. Zutshi M, Salcedo LB, Zaszczurynski PJ, Hull TL, Butler RS, Damaser MS. Effects of sphincterotomy and pudendal nerve transection on the anal sphincter in a rat model. Dis Colon Rectum. 2009. 52:1321–1329.
15. Kajbafzadeh AM, Elmi A, Talab SS, Esfahani SA, Tourchi A. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon Rectum. 2010. 53:1415–1421.
16. Frudinger A, Kolle D, Schwaiger W, Pfeifer J, Paede J, Halligan S. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut. 2010. 59:55–61.
17. Kang SB, Lee TG. Muscle regeneration: research for the treatment of fecal incontinence. J Korean Soc Coloproctol. 2010. 26:1–7.
18. Lim SM, Lee HJ, Oh SH, Kim JM, Lee JH. Novel fabrication of PCL porous beads for use as an injectable cell carrier system. J Biomed Mater Res B Appl Biomater. 2009. 90:521–530.
19. Oh SH, Kim IG, Lee JY, Lee JY, Lee JH. Bioactive porous beads as an injectable urethral bulking agent: their in vitro evaluation on smooth muscle cell differentiation. Tissue Eng Part A. 2011. 17:655–664.
20. Kim IG, Oh SH, Lee JY, Lee JY, Lee JH. Bioactive porous beads as an injectable urethral bulking agent: in vivo animal study for the treatment of urinary incontinence. Tissue Eng Part A. 2011. 17:1527–1535.
21. Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994. 125:1275–1287.
22. Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008. 29:2899–2906.
23. Saihara R, Komuro H, Urita Y, Hagiwara K, Kaneko M. Myoblast transplantation to defecation muscles in a rat model: a possible treatment strategy for fecal incontinence after the repair of imperforate anus. Pediatr Surg Int. 2009. 25:981–986.
24. Nijhuis PH, van den Bogaard TE, Daemen MJ, Baeten CG. Perianal injection of polydimethylsiloxane (Bioplastique implants) paste in the treatment of soiling: pilot study in rats to determine migratory tendency and locoregional reaction. Dis Colon Rectum. 1998. 41:624–629.
25. Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev. 2010. (5):CD007959.
26. Alameddine HS, Louboutin JP, Dehaupas M, Sebille A, Fardeau M. Functional recovery induced by satellite cell grafts in irreversibly injured muscles. Cell Transplant. 1994. 3:3–14.
27. Guettier-Sigrist S, Coupin G, Warter JM, Poindron P. Cell types required to efficiently innervate human muscle cells in vitro. Exp Cell Res. 2000. 259:204–212.
28. Culver PJ, Rattan S. Genesis of anal canal pressures in the opossum. Am J Physiol. 1986. 251(6 Pt 1):G765–G771.
29. Kimura J. Electrodiagnosis in disease of nerve and muscle. 2001. Oxford: Oxford Univ ersity Press.
30. Yazici I, Ayhan S, Elmas C, Temucin C, Atabay K. Motor neurotization by segmental epineurectomy and implantation: lateral muscular neurotization. J Reconstr Microsurg. 2008. 24:435–442.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download