Abstract
Purpose
Free tumor cells in peritoneal fluid in patients with pancreatic cancer may have prognostic significance but there are few reports on methods for the effective detection of free tumor cells. The aims of this study were to identify free cancer cells in peritoneal fluid with fluorescent in situ hybridization (FISH) technique and to investigate its prognostic significance.
Methods
Twenty-eight patients with resectable pancreatic cancer who underwent surgical resection were included. Peritoneal washing and peritoneal drainage fluid were examined by FISH for p53 deletion.
Results
Among the study subjects, the R0 resection rate was 75%. None of the patients had positive cytology with Papanicolaou's method. p53 deletion was detected in 9 peritoneal washings (32.1%) and in 5 peritoneal drainage fluids (17.9%). After a median of 18 months of follow-up, 25 patients (89.3%) experienced recurrence and 14 patients (50.0%) had peritoneal seeding. Patients with p53 deletion detected in the peritoneal drainage fluid had positive radial margin (60.0% vs. 17.4%, P = 0.046) more frequently and a lower peritoneal metastasis free survival (median, 11.1 months vs. 30.3 months; P = 0.030). Curative resection (P < 0.001) and p53 deletion in peritoneal drainage fluid (P = 0.030) were independent risk factors of peritoneal metastasis free survival after multivariate analysis.
Conclusion
FISH technique detects free cancer cells with higher sensitivity compared to Papanicolaou's method. p53 deletion detected in peritoneal drainage fluid is correlated with positive radial resection margin and results in early peritoneal seeding. Patients with p53 deletion in peritoneal drainage fluid need more aggressive adjuvant treatment.
Peritoneum is one of the most frequent sites of metastasis in pancreatic cancer [1]. However, it is difficult to detect microscopic disease spread in the peritoneum during the operation unless it forms visible lesions in the peritoneum. Peritoneal cytology has been used as clinical parameters for prognostication of gastric [2] or gynecologic malignancies [3]. However, the role and prognostic relevance of peritoneal cytology for pancreatic cancer have not been well defined. Some have reported positive peritoneal cytology as a poor prognostic factor of pancreatic cancer [4,5], but others have regarded positive cytology without any other evidence of distant metastasis not as an independent prognostic factor for overall survival of pancreatic cancer [6-8].
Moreover, there has been limited number of studies dealing with postoperative peritoneal drainage fluid for the evaluation of free cancer cells in pancreatic cancer. To detect minimal residual disease after R0 resection, peritoneal drainage fluid can be used as an important material for the evaluation of free cancer cells in the peritoneum.
Furthermore, detection methods for peritoneal micrometastasis are another challenge. Most of the previous studies have used Papanicolaou's method for the detection of free cancer cells in peritoneal fluids [4-10]. However, conventional cytologic examination has limited sensitivity for detecting peritoneal micrometastasis, and it can have biased results according to pathologists. A recent study reported increased sensitivity of cytologic examination using reverse transcription-polymerase chain reaction for the peritoneal fluid of pancreatic cancer patients [11], or using fluorescent in situ hybridization (FISH) for brush cytologic examination for patients with malignant biliary obstruction [12] and pancreatic cancer [13].
In this study, the authors investigated the prognostic relevance of cytologic examination of peritoneal washing and postoperative peritoneal drainage fluid using the FISH technique in potentially resectable pancreatic cancer patients.
From 2005 to 2006, patients with potentially resectable pancreatic adenocarcinoma undergoing pancreatectomy were prospectively enrolled. The demographic and pathologic characteristics of the patients including follow-up data on recurrence, FISH examination for p53 deletion in peritoneal washing fluid and peritoneal drainage fluid were prospectively collected.
Peritoneal washing fluid and peritoneal drainage fluid were collected according to the following protocol. At the beginning of the operation, peritoneal lavage with 1 L of normal saline was performed and the lavage fluid was collected in a sterile bottle mixed with same amount of Saccamano's cytology fixative. At the end of the operation, 3 Silastic peritoneal drainage tubes were placed at the operation field. At postoperative day 2, 100 mL of peritoneal drainage fluid was collected in the same amount of Saccamano's cytology fixative. For conventional cytologic examination, after centrifugation of the collected fluid for 3 minutes at 2,000 rpm, direct smears were prepared and fixed in 95% ethanol and were stained with Papanicolaou's method. All slides were reviewed by an experienced cytopathologist.
FISH examination was performed as follows. After centrifugation of the collected fluid for 5 minutes at 1,000 rpm, the supernatant was removed and the pellet was resuspended with 1 × phosphate buffered saline. The suspension was centrifuged for 5 minutes at 1,000 rpm, and after removal of the supernatant, 5 mL of 0.075 M KCl was added. After incubation for 25 minutes in a 37℃ water bath, 1 mL of Carnoy fixative was added. The suspension was centrifuged for 5 minutes at 1,000 rpm and the supernatant was removed. The pellet was resuspended with 5 mL of Carnoy fixative and direct smears were prepared at a cellular density of 1 × 106/mL. A dried slide was incubated with 50 mL of 0.1% NP-40/2×SSC for 30 minutes in a 37℃ water bath and dehydrated with 70%, 85%, and 100% ethanol for 3 minutes each. Under protection from light, a FISH probe (LSI p53 [17p13.1] SpectrumOrange probe; Vysis Inc., Downers Grove, IL, USA) was hybridized with the prepared slide. After 3 minutes of denaturation at 75℃, the slide was incubated for 24 hours at 39℃. After hybridization, the slide was incubated two times with 50% formamide/2×SSC for 10 minutes at 46℃, 2×SSC for 10 minutes at 46℃, and 0.1% NP-40/2×SSC for 5 minutes at 46℃. For counterstaining, 10ul of DAPI was added to the slide. With a cutoff value of 10%, the slide was examined with a fluorescent microscope.
The demographic findings of the study subjects are listed in Table 1. The mean age of the study subjects was 60.1 years and the male to female ratio was 2.5 to 1. Surgery with curative intent was performed in 25 patients (89.3%). None of the patients had malignant cells detected with Papanicolaou's method. The detection rates for p53 deletion in the peritoneal washing fluid and peritoneal drainage fluid were 32.1% (n = 9, Fig. 1) and 17.9% (n = 5), respectively. p53 deletion was not detected in any of the peritoneal washing or peritoneal drainage fluids in 13 patients (46.4%), and p53 deletion was detected in both the peritoneal washing and peritoneal drainage fluid in 1 patient (3.6%). Twenty-three patients (82.1%) received adjuvant treatment. Fourteen patients (77.8%) had concurrent chemoradiation therapy followed by gemcitabine based maintenance chemotherapy. One patient (5.6%) had radiation therapy only, and 5 patients (27.8%) had gemcitabine based chemotherapy only. All of the patients were followed up for survival analysis for at least 5 years. Twenty-three patients (82.1%) died during the follow-up period, and 25 patients (89.3%) experienced recurrence. The pathologic characteristics of the patients are presented in Table 2. Lymph node metastasis was identified in 20 patients (71.4%) and 7 patients (25.0%) had microscopic positive resection margin.
Nine patients (32.1%) had p53 deletion detected in the peritoneal washing fluid. Patients with p53 deletion detected in the peritoneal washing fluid had comparable T stage (T3, 9/9 vs. 18/19, P = 1.000), lymph node metastasis (6/9 vs. 14/19, P = 1.000), perineural invasion (9/9 vs. 15/19, P = 0.273), and lymphovascular invasion (6/9 vs. 11/19, P = 0.937) compared with those without p53 deletion. Patients with p53 deletion detected in the peritoneal washing fluid had comparable radial margin positive rate (4/9 vs. 4/19, P = 0.405) or recurrence rate (8/9 vs. 17/19, P = 1.000) between those with or without p53 deletion detected in the peritoneal washing fluid.
Five patients (17.9%) had p53 deletion detected in the peritoneal drainage fluid (Table 3). Patients with p53 deletion detected in the peritoneal fluid had positive radial margin more frequently (3/5 vs. 4/23, P = 0.046). The overall recurrence rate was comparable between those with or without p53 deletion detected in the peritoneal drainage fluid (5/5 vs. 20/23, P = 0.284).
Eight patients had p53 deletion in the peritoneal washing fluid only. Comparing these patients with 5 patients who had p53 deletion detected in the peritoneal drainage fluid, T3 stage (100% vs. 100%, P = 0.110), lymph node metastasis rate (62.5% vs. 100%, P = 0.376), perineuralinvasion rate (100% vs. 100%, P = 0.110), lymphovascular invasion rate (62.5% vs. 60.0%, P = 0.620) were comparable between two groups. Radial resection margin positive rate was higher in patients with p53 deletion in peritoneal fluid than those with p53 deletion in peritoneal washing fluid only (100% vs. 25.0%, P = 0.039).
Overall median survival of the study subjects was 17.8 months (95% confidence interval, 13.8 to 21.8). According to peritoneal washing fluid analysis, overall survival (3-year survival rate, 33.3% vs. 21.1%; P = 0.961), disease free survival (3-year disease free survival rate, 11.1% vs. 10.5%; P = 0.742), time to local recurrence (median, 17.7 vs. not reached; P = 0.657), time to liver metastasis (median, 11.4 vs. not reached; P = 0.343), and time to peritoneal metastasis (median, 26.6 months vs. 30.3 months, P = 0.598) were comparable between those with or without p53 deletion.
According to the peritoneal drainage fluid analysis, overall survival (3-year survival rate, 0% vs. 30.4%; P = 0.223), disease free survival (3-year disease free survival rate, 0% vs. 13.0%; P = 0.277), time to local recurrence (median, 11.0 months vs. 53.6 months; P = 0.198), and time to liver metastasis (median, 11.4 months vs. 18.8 months; P = 0.797) were comparable between those with or without p53 deletion, but time to peritoneal metastasis was shorter in patients with p53 deletion than in those without p53 deletion (median, 11.1 months vs. 30.3 months; P = 0.030) (Fig. 2).
Disease free survival of resectable pancreas cancer was associated with surgery with curative intent (P = 0.015) or lymphovascular invasion (P = 0.058). Peritoneal metastasis free survival was associated with surgery with curative intent (P < 0.001), p53 deletion detected in peritoneal drainage fluid (P = 0.030), and positive resection margin (P = 0.093) (Table 4). From the multivariate analysis, palliative resection and p53 deletion detected in the peritoneal drainage fluid were independent factors for peritoneal metastasis free survival (Table 5).
Most pancreatic cancers have cytogenetic alterations. Human pancreatic cancers usually have an increased copy number of c-myc, a decreased copy number of p16, deletion of p53, loss of chromosome 18q and gain of chromosome 20q [13]. Our previous study showed that all of the pancreatic cancer tissues had p53 deletions detected by the FISH technique [13]. As a consequence, the authors selected p53 deletion as a cancer cell detection marker in peritoneal washing and peritoneal drainage fluid.
The clinical impact of peritoneal cytology for pancreatic cancer is under debate. Poor survival outcomes in patients with positive peritoneal cytology have been suggested [7,11] but some have reported that positive peritoneal cytology alone does not contraindicate radical surgery in patients with resectable pancreatic cancer [6,8,14,15]. Moreover, positive cytology is not a contraindication of curative surgery for colon cancer [16] or gynecological cancers [17]. In this study, there were no patients with positive cytology using conventional cytologic examinations. Peritoneal washing fluid analysis with the FISH technique was not related with overall or disease free survival and local or systemic recurrence. However, peritoneal drainage fluid analysis with the FISH technique was related with early peritoneal metastasis; therefore, microscopic residual disease detected with the FISH technique may have had an important role in the prognosis of cases with resectable pancreatic cancer which did not have visible metastasis at the time of the operation. Previously, postoperative cytology for lung cancer [18] or esophageal cancer resection [19] showed increased recurrence and shorter survival based on a higher incidence of distant metastasis. In pancreatic cancer, Ishikawa et al. [20] first reported the prognostic relevance of drain cytology for pancreatic cancer which revealed local recurrence was more frequent in patients with positive drain cytology. However, in this study, neither the rate of local recurrence nor the time to local recurrence was affected by p53 deletion detected in peritoneal drainage fluid. Instead, p53 deletion detected in peritoneal drainage fluid was associated with early peritoneal metastasis. Pancreatic cancers usually infiltrate into retroperitoneal nerve plexuses [21] or lymphatic tissues [22] on a microscopic level although macroscopic tumors are confined to the pancreas. The data of this study suggest that microscopic residual disease that outflows from the vessels and lymphatics at the operation bed flows into the peritoneal cavity, which results in peritoneal metastasis. Moreover, since the survival of pancreatic cancer patients is dependent on distant metastasis rather than local recurrence, prediction of peritoneal metastasis has more prognostic relevance than local recurrence.
Traditionally, the prognostic value of the cytologic examination of peritoneal washing fluid in pancreatic cancer has been evaluated by Papanicolaou's method. The detection rate of malignant cells in peritoneal washings from potentially resectable pancreatic cancer using Papanicolaou's method has been reported to range from 5% to 32% [4-10]. In this study, none of the patients with resectable pancreatic adenocarcinoma had positive cytology for both peritoneal washing and peritoneal drainage fluid. Because this study included higher portion of patients who underwent curative resection, positive cytology rate would be lower than other reports. Moreover, limitation in study subject number would have reduced positive cytology rate. On the other hand, p53 deletion was detected with the FISH technique in 32.1% of the peritoneal washing fluids and 17.9% of the peritoneal drainage fluids. p53 deletion detected in the peritoneal washing fluid was not associated with tumor stage or recurrence. However, p53 deletion detected in peritoneal drainage fluid was associated with positive radial margin which reflects the presence of microscopic residual disease. Moreover, p53 deletion detected in peritoneal drainage fluid was associated with early peritoneal metastasis. Therefore, the low sensitivity of the conventional cytologic examination can be overcome with the FISH technique which can identify microscopic residual disease which can predict early recurrence.
In conclusion, while none of the patients had positive peritoneal cytology with Papanicolaou's method, the FISH technique detected p53 deletion in 32.1% of the peritoneal washing fluids and 17.9% of the peritoneal drainage fluids. The FISH technique had higher sensitivity in detecting free cancer cells compared to the conventional cytologic examination. With the FISH technique, p53 deletion detected in the peritoneal washing fluid was not associated with the prognosis of the patients but p53 deletion detected in the peritoneal drainage fluid was associated with positive radial resection margin more frequently and early peritoneal metastasis. Detecting p53 deletion with the FISH technique is an effective method to identify microscopic residual disease of resectable pancreatic cancer after curative intended resection. More aggressive systemic chemotherapy should be performed for patients with p53 deletion detected in peritoneal drainage fluid.
Figures and Tables
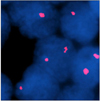 | Fig. 1Fluorescent in situ hybridization analysis of p53 using single color probe. A normal cell is shown with two orange signals. Heterozygous deletion of p53 is shown with one orange signal in a pancreatic cancer cell detected in peritoneal fluid. |
 | Fig. 2Peritoneal metastasis-free survival according to p53 deletion detected by fluorescent in situ hybridization in peritoneal drainage fluid. Median survival was shorter in patients with p53 deletion than those without (11.1 months vs. 30.3 months, P = 0.030). |
ACKNOWLEDGEMENTS
This study was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-E00153) and a grant from Seoul National University Hospital (No. 04-2004-0510).
References
1. Kang MJ, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Comparison of the long-term outcomes of uncinate process cancer and non-uncinate process pancreas head cancer: poor prognosis accompanied by early locoregional recurrence. Langenbecks Arch Surg. 2010. 395:697–706.
2. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011. 14:101–112.
3. American Joint Committee on Cancer. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. Ovary and primary peritoneal carcinoma. AJCC cancer staging manual. 2010. 7th ed. New York: Springer;493–506.
4. Leach SD, Rose JA, Lowy AM, Lee JE, Charnsangavej C, Abbruzzese JL, et al. Significance of peritoneal cytology in patients with potentially resectable adenocarcinoma of the pancreatic head. Surgery. 1995. 118:472–478.
5. Merchant NB, Conlon KC, Saigo P, Dougherty E, Brennan MF. Positive peritoneal cytology predicts unresectability of pancreatic adenocarcinoma. J Am Coll Surg. 1999. 188:421–426.
6. Konishi M, Kinoshita T, Nakagohri T, Inoue K, Oda T, Takahashi S. Prognostic value of cytologic examination of peritoneal washings in pancreatic cancer. Arch Surg. 2002. 137:475–480.
7. Ferrone CR, Haas B, Tang L, Coit DG, Fong Y, Brennan MF, et al. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg. 2006. 10:1347–1353.
8. Yamada S, Takeda S, Fujii T, Nomoto S, Kanazumi N, Sugimoto H, et al. Clinical implications of peritoneal cytology in potentially resectable pancreatic cancer: positive peritoneal cytology may not confer an adverse prognosis. Ann Surg. 2007. 246:254–258.
9. Jimenez RE, Warshaw AL, Fernandez-Del Castillo C. Laparoscopy and peritoneal cytology in the staging of pancreatic cancer. J Hepatobiliary Pancreat Surg. 2000. 7:15–20.
10. Martin JK Jr, Goellner JR. Abdominal fluid cytology in patients with gastrointestinal malignant lesions. Mayo Clin Proc. 1986. 61:467–471.
11. Kelly KJ, Wong J, Gladdy R, Moore-Dalal K, Woo Y, Gonen M, et al. Prognostic impact of RT-PCR-based detection of peritoneal micrometastases in patients with pancreatic cancer undergoing curative resection. Ann Surg Oncol. 2009. 16:3333–3339.
12. Barr Fritcher EG, Caudill JL, Blue JE, Djuric K, Feipel L, Maritim BK, et al. Identification of malignant cytologic criteria in pancreatobiliary brushings with corresponding positive fluorescence in situ hybridization results. Am J Clin Pathol. 2011. 136:442–449.
13. Yoon YS, Lee DS, Min HC, Jang JY, Lee SE, Hwang DW, et al. Analysis of molecular cytogenetic alteration of pancreatic cancer identified by fluorescent in situ hybridization (FISH) and its clinical significance. Korean J Hepatobiliary Pancreat Surg. 2008. 12:75–85.
14. Yachida S, Fukushima N, Sakamoto M, Matsuno Y, Kosuge T, Hirohashi S. Implications of peritoneal washing cytology in patients with potentially resectable pancreatic cancer. Br J Surg. 2002. 89:573–578.
15. Meszoely IM, Lee JS, Watson JC, Meyers M, Wang H, Hoffman JP. Peritoneal cytology in patients with potentially resectable adenocarcinoma of the pancreas. Am Surg. 2004. 70:208–213.
16. Vogel P, Ruschoff J, Kummel S, Zirngibl H, Hofstadter F, Hohenberger W, et al. Prognostic value of microscopic peritoneal dissemination: comparison between colon and gastric cancer. Dis Colon Rectum. 2000. 43:92–100.
17. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. Version 3. 2012 [Internet]. c2012. cited 2012 Aug 27. Fort Wathington: NCCN;Available from: http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
18. Higashiyama M, Doi O, Kodama K, Yokouchi H, Tateishi R, Horai T, et al. Pleural lavage cytology immediately after thoracotomy and before closure of the thoracic cavity for lung cancer without pleural effusion and dissemination: clinicopathologic and prognostic analysis. Ann Surg Oncol. 1997. 4:409–415.
19. Doki Y, Kabuto T, Ishikawa O, Ohigashi H, Sasaki Y, Yamada T, et al. Does pleural lavage cytology before thoracic closure predict both patient's prognosis and site of cancer recurrence after resection of esophageal cancer? Surgery. 2001. 130:792–797.
20. Ishikawa O, Wada H, Ohigashi H, Doki Y, Yokoyama S, Noura S, et al. Postoperative cytology for drained fluid from the pancreatic bed after "curative" resection of pancreatic cancers: does it predict both the patient's prognosis and the site of cancer recurrence? Ann Surg. 2003. 238:103–110.
21. Ohigashi H, Ishikawa O, Sasaki Y, Yamada T, Furukawa H, Imaoka S, et al. K-ras point mutation in the nerve plexuses around the superior mesenteric artery in resectable adenocarcinoma of the pancreatic head: distribution pattern and related factors. Arch Surg. 2000. 135:1450–1455.
22. Demeure MJ, Doffek KM, Komorowski RA, Wilson SD. Adenocarcinoma of the pancreas: detection of occult metastases in regional lymph nodes by a polymerase chain reaction-based assay. Cancer. 1998. 83:1328–1334.




 ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download