Abstract
Purpose
Regional lymph node (LN) metastases are detected in 57-85% of patients with papillary thyroid carcinoma (PTC) and are associated with increased tumor recurrence. However, the management of lymphatic disease in patients with PTC has been ongoing source of debate. We have prospectively assessed the usefulness and accuracy of sentinel LN (SLN) biopsy for the detection of LN metastases in patients with PTC on preoperative imaging using single photon emission computed tomography/computed tomography (SPECT/CT) and 99mTc phytate.
Methods
We prospectively assessed 39 patients with PTC who had risk factors for recurrence or with the necessity of intraoperative LN sampling for suspicious LN metastases on preoperative imaging from August 2010 to March 2011. The patients underwent preoperative lymphoscintigraphy and SPETC/CT and intraoperative SLN biopsy (SLNB).
Results
99mTc lymphoscintigraphy and SPECT/CT localized SLN in 38 patients (97.4%), with the gamma probe identifying 2.15 mean SLNs in the lateral neck of the 39 patients. Skip metastasis was found in one patient, and lateral compartment LN metastasis in 17 (43.5%). The sensitivity, specificity, and accuracy of SLNB for lateral compartment LN metastasis were 88.2%, 100%, and 94.8%, respectively. SLNB was more accurate and useful for lateral than for central compartment LN metastasis.
Conclusion
SPECT/CT improved SLN detection and anatomical localization compared with lymphoscintigraphy. SLNB in patients with risk factors for recurrence or the necessity of intraoperative LN sampling for suspected LN metastases on preoperative imaging was accurate in detecting LN metastases and may help in deciding whether to perform lateral compartment dissection in patients with PTC.
The incidence of thyroid cancer has increased continuously worldwide [1,2]. Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, accounting for up to 80% of primary thyroid cancers [3]. Cervical lymph node (LN) metastasis frequently occurs in patients with PTC, and up to 90% of patients with new thyroid malignancies are found to have LN metastases [4]. Since LN metastasis is a risk factor for recurrence [5]. The detection of such metastases prior to or during initial surgery may affect recurrence and patients survival.
Although ultrasonography has been shown to be the most useful tool for preoperatively detecting cervical LN metastases, its accuracy has been found to vary widely, from 40% to 90%, and to be operator-dependent [6]. Use of a combinatioin of ultrasonography and a second method, such as intraoperative sentinel LN biopsy (SLNB), may identify LN metastases more [7-9].
SLNB is the gold standard for detecting LN metastases in patients with melanoma and breast cancer. This technique has also been utilized in patients with thyroid cancer, using vital blue dye [10], or a radioisotope with favorable results observed in patients with PTC [11,12]. A complementary method, preoperative lymphoscintigraphy defines the drainage basins and SLN locations of individual tumors [13].
However, because LN drainage in the head and neck is unpredictable and ambiguous, preoperative lymphoscintigraphic SLN mapping is important for tailoring the surgical field in individual patients [14]. Since planar scintigraphy did not provide the precise location of SLN, due to poor anatomic information, single photon emission computed tomography/computed tomography (SPECT/CT) has been used to preoperatively map SLN in patients with head, neck and gynecologic cancer. We have, therefore, investigated the usefulness and accuracy of SLNB, as detected by preoperative SPECT/CT with technetium-99m phytate, of locoregional LN metastases in patients with PTC. To our knowledge, this is the first report on the use of SPECT/CT for localizing SLN in PTC.
From July 2010 to March 2011, 39 patients with differentiated PTC underwent SLNB of the central and lateral neck compartments in our institution. All patients had been preoperatively diagnosed with PTC by fine needle aspiration (FNA) biopsy and had risk factors for recurrence or required intraoperative LN sampling for suspected LN metastases on preoperative imaging. No patient had a history of either thyroid or neck surgery. Patients with other types of thyroid malignancies were excluded. The study was approved by the Institutional Review Board of our institution and written informed consent was obtained from each patient.
Twenty MBq 99mTc phytate was injected into each tumor, using a 26-gauge syringe. Early lymphoscintigraphy and SPECT/CT were performed 10 minutes later. Delayed lymphoscintigraphy and SPECT/CT were performed, 2 hours after injection. Surgery was performed 2 to 4 hours after injection. Anterior planar images were obtained during lymphoscintigraphy. SPECT/CT emission/transmission was performed using a hybrid system consisting of a dual-head gamma camera with a low-dose X-ray tube installed in its gantry (Infinia Hawkeye 4 SPECT-CT, GE Healthcare, Waukesha, WI, USA). This system allows both transmission and emission acquisitions without changing the patient's position. SPECT acquisition parameters for SLN detection included a matrix size of 128 × 128, 180 degree in anterior L-moderotation, and a 4 degree angle step with 25s time frame. Transmission data of the patient were corrected and reconstructed using the filtered back-projection to produce cross-sectional attenuation of the imaged tissue. The SPECT and CT images were fused on the Xeleris Function Imaging Workstation version 2.1507. The hottest node found positive on both preoperative lymphoscintigraphy and SPECT/CT was considered the sentinel LN, as previously reported [15].
Under general anesthesia, a standard, transverse, low-collar skin incision was made, and the myocutaneous flap was lifted. A fascial incision was made between the strap musculature and the sternocleidomastoid muscle, exposing the ipsilateral jugular vein. The SLN was harvested using the intraoperative hand-held probe (Neoprobe 2000, Johnson & Johnson Medical, Hamburg, Germany) and sent for both frozen and permanent sections. Total thyroidectomy was completed subsequently and all LNs of the central compartment were dissected. Modified radical neck dissection (mRND) was performed only in patients with positive LNs, and the nodes were labeled and sent for permanent histological section. All patients underwent compartment dissection at cervical levels II, III and IV.
The radioactivity counts of the lymphatic basin were assessed before and after excision of each node, and the radioactivity of each excised node was recorded after excision. Any node that had a count of at least 10% of the radioactivity of the hottest node was excised.
Pathologic specimens were stained with hematoxylin and eosin and were observed under light microscopy. The sensitivity, specificity, accuracy, and positive predictive value (PPV) and negative predictive value (NPV) of SLN biopsies, were calculated. Other variables were investigated using t-test and chi-square tests. All statistical calculations were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). Postsurgical morbidity information was obtained from follow-up clinical and laboratory data.
We assessed 39 patients (9 males and 30 females), of mean age 45 years (range, 30 to 70 years). Mean (±standard deviation) tumor size was 1.30 ± 0.73 cm. Bilateral jugular LN metastases were found in 4 patients, and unilateral jugular LN metastases in 13. The demographic and clinical characteristics of these patients, as well as their treatments and morbidities, are summarized in Table 1.
A SLN was detected in 38 patients (97.4%). The mean number of SLNs per patients was 4.73 ± 3.06 (range, 0 to 12), the mean number of lateral compartments, in which SLN was detected, was 2.15 ± 0.921 (range, 0 to 4), and the mean number of SLNs per lateral compartment was 2.22 ± 1.36 (range, 0 to 6). The sensitivity, specificity, accuracy, PPV and NPV of SLNB for lateral LN metastasis were 88.2%, 100%, 94.8%, 100%, and 91.6%, respectively (Table 2). The sensitivity, accuracy and NPV for lateral LN metastasis were higher than those for central LN metastasis (Table 3).
The association between the tumor sites and the pattern of LN metastasis in the ipsilateral compartment was not statistically significant (P = 0.055) (Fig. 1). In the patient with the skip metastasis, the primary tumor was located in the upper portion of the thyroid gland.
When we calculated the concordance rate based on cervical levels between suspicious LNs on US and SPECT/CT, the mean concordance rate was 27.9%.
When we compared preoperative US-guided FNA with intraoperative SLNB in 11 patients, the sensitivity of SLNB was higher than that of preoperative FNA at lateral neck (88.2% vs. 33.3%) and central LN metastases in 7 patients among them were diagnosed with SLNB only.
PTC is a representative histological type of thyroid cancer. It shows a mild behavior and has an excellent prognosis. However, LN metastases are frequently detected, primarily in cervical LN. Cervical LN metastases have been associated with a higher incidence of recurrence [16,17]. Although radioiodine treatment may reduce recurrence, 131I ablation was effective in only 10% of high-risk patients according to the AMES (age, metastasis to distant sites, extrathyroidal invasion, size) classification, and was not effective in 30% of low-risk patients [18].
Ultrasonography is useful in the preoperative assessment of cervical LN metastasis in patients with PTC [6]. However, a diagnosis of LN metastasis is difficult, because most patients with PTC have multiple, very small nodes <5 mm in diameter and metastatic LNs may show no change on serial imaging over several years [3]. Therefore the accuracy of ultrasonography in detecting cervical LN metastasis is not very high. The accuracy of loss of fatty hilum, round shape, calcification, cystic change or abnormal vascularity has been reported to range from 51% to 77% [19]. Ultrasonography may be complemented by measuring Tg in FNA (FNA-Tg) or by LN sampling during surgery. However, false positive or false negative FNA-Tg results have been reported, although their frequencies were low, and FNA-Tg cannot be performed in patients with obstruction of the carotid artery [20]. Moreover, it may be impossible to dissect LNs targeted during intraoperative sampling, because of the necessity to perform blunt dissection without anatomic landmarks. Therefore, in these patients, SLNB may accurately diagnose LN metastases.
To date, indication for SLNB have not been established. The recurrence rate in cervical LNs has been reported to range from 5.8% to 10.7% [6,21,22], with risk factors for recurrence including age, tumor size, extrathyroidal extension, LN metastasis and sex, et al. [6,21]. For example, recurrence rates were reported to be higher in patients <30 years of age and patients with larger sized tumors, cervical LN metastasis, local invasion [21]. Another study reported that risk factors for recurrence were age >54 years, LN metastasis and tumor size >4 cm [6].
Prophylactic mRND has been recommended to prevent recurrence in cervical LNs [6,23]. For example, patients with LN metastasis or, capsular invasion and woman >60 years of age were reported to benefit from mRND [23]. Similarly, prophylactic mRND may be beneficial in patients with two or more of the following characteristics: male gender, age ≥55 years, maximal tumor diameter >3 cm, and massive extrahyroid extension of the tumor [6]. Due to the high morbidity rates associated with mRND, however, the gold standard of lateral LN management is not prophylactic, but therapeutic mRND.
Therefore selective SLNB may determine whether therapeutic mRND should be utilized in patients <30 or >54 years of age, with tumor size >3 cm in diameter, or with local invasioin, or to reduce false positive or false negative FNA cytology or FNA-Tg results.
We assessed the usefulness and accuracy of SLNB in patients with risk factors for recurrence or requiring intraoperative LN sampling for suspected LN metastases on preoperative SPECT/CT. We found that the sensitivity, specificity, accuracy, and PPV of SLNB for lateral and central LN metastases were comparable to results from previous reports that did not utilize SPECT/CT, with sensitivity ranged from 77.7% to 91% and accuracies from 90% to 95.3% [24,25]. In addition, the high resolution and anatomic information of SLN provided by SPECT/CT made intraoperative gamma probing easier, compared with information provided by palpation and inspection [15].
In addition, SPECT/CT may also show pathway of lymph basins. We observed one patients with a lymph basin extending from the right jugular LN at cervical level II to the right retropharyngeal LN (Fig. 2), and another with a basin extending from the right paratracheal to the right high mediastinal LN (Fig. 3). However, the distribution of SLNs was not similar to that of metastatic LNs in the lateral neck. The ipsilateral distribution of SLNs showed the highest frequency at level IV, whereas the distribution of metastatic LNs showed the highest frequency at level III.
Generally, lymphatic metastasis from differentiated PTC occurs in a stepwise manner from the LNs in the tracheoesophageal groove to the LNs in the jugular chain, including the supraclavicular fossa [26]. Following the initial metastases in the central compartment, LN metastases were observed in the deep inferior and lateral cervical nodes, with no relation with tumor location [27]. We also found that the pattern of LN metastasis is not related to the primary tumor site. However, a skip metastasis, defined as lateral without central LN metastasis and observed in 7.7% to 15.3% of patients [28-30], was observed in only one patients with PTC in the upper portion of thyroid gland. Therefore, the relationship between tumor location and the pattern of LN metastasis requires further investigation.
Although SLNB is useful to identify the LN metastasis, it has two demerits. Unnecessary lateral LN dissection can be performed in spite of subclinical LN metastases, which are not related with recurrence, because the frequency of occult LN metastases of papillary thyroid cancer is 40% to 90% [10]. In addition, SLNB takes long surgical time because two or more SLN are identified in most of our patients (Table 1) dut to the rich lymphatic system of the thyroid gland. Therefore, routine SLNB may result in longer surgical time and hospitalization with increased surgical morbidity.
In conclusion, SLNB using SPECT/CT with technetinum-99m may be useful in accurately assessing LN stage during surgery patients with risk factors for recurrence or those requiring intraoperative LN sampling for suspected LN metastases on preoperative imaging. However, further studies are required for appropriate method of SLNB, by which the distribution of SLNs can be closer to those of metastatic LNs for the sake of avoiding unnecessary dissection in 67% of the patients without LN metastasis.
Figures and Tables
Fig. 1
Frequency of tumor sites and metastatic lymph node distribution (P = 0.055). 1, upper; 2, middle and lower.

Fig. 2
Single photon emission computed tomography/computed tomography of the basin from the right jugular lymph node (LN) of cervical level II to the right retropharyngeal LN.
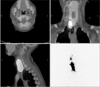
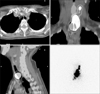
Fig. 3
Single photon emission computed tomography/computed tomography of the basin from the right paratracheal lymph node (LN) to the right high mediastinal LN.
References
1. Ronckers CM, McCarron P, Ron E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer. 2005. 117:281–288.
2. National Cancer Information Center. Cancer facts & figures 2012 in the republic of Korea [Internet]. c2012. cited 2013 Mar 20. Goyang: National Cancer Center;Available from: http://www.cancer.go.kr/mbs/cancer/jsp/album/gallery.jsp?spage=1&boardId=31817&boardSeq=179615&mcategoryId=&id=cancer_050207000000.
3. King AD. Imaging for staging and management of thyroid cancer. Cancer Imaging. 2008. 8:57–69.
4. Machens A, Hinze R, Thomusch O, Dralle H. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg. 2002. 26:22–28.
5. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994. 97:418–428.
6. Ito Y, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg. 2007. 31:2085–2091.
7. Alex JC, Sasaki CT, Krag DN, Wenig B, Pyle PB. Sentinel lymph node radiolocalization in head and neck squamous cell carcinoma. Laryngoscope. 2000. 110(2 Pt 1):198–203.
8. Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993. 2:335–339.
9. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992. 127:392–399.
10. Kelemen PR, Van Herle AJ, Giuliano AE. Sentinel lymphadenectomy in thyroid malignant neoplasms. Arch Surg. 1998. 133:288–292.
11. Gallowitsch HJ, Mikosch P, Kresnik E, Starlinger M, Lind P. Lymphoscintigraphy and gamma probe-guided surgery in papillary thyroid carcinoma: the sentinel lymph node concept in thyroid carcinoma. Clin Nucl Med. 1999. 24:744–746.
12. Sahin M, Yapici O, Dervisoglu A, Basoglu T, Canbaz F, Albayrak S, et al. Evaluation of lymphatic drainage of cold thyroid nodules with intratumoral injection of Tc-99m nanocolloid. Clin Nucl Med. 2001. 26:602–605.
13. Catarci M, Zaraca F, Angeloni R, Mancini B, de Filippo MG, Massa R, et al. Preoperative lymphoscintigraphy and sentinel lymph node biopsy in papillary thyroid cancer: a pilot study. J Surg Oncol. 2001. 77:21–24.
14. Even-Sapir E, Lerman H, Lievshitz G, Khafif A, Fliss DM, Schwartz A, et al. Lymphoscintigraphy for sentinel node mapping using a hybrid SPECT/CT system. J Nucl Med. 2003. 44:1413–1420.
15. Carcoforo P, Feggi L, Trasforini G, Lanzara S, Sortini D, Zulian V, et al. Use of preoperative lymphoscintigraphy and intraoperative gamma-probe detection for identification of the sentinel lymph node in patients with papillary thyroid carcinoma. Eur J Surg Oncol. 2007. 33:1075–1080.
16. McHenry CR, Rosen IB, Walfish PG. Prospective management of nodal metastases in differentiated thyroid cancer. Am J Surg. 1991. 162:353–356.
17. Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994. 18:559–567.
18. Cady B, Sedgwick CE, Meissner WA, Wool MS, Salzman FA, Werber J. Risk factor analysis in differentiated thyroid cancer. Cancer. 1979. 43:810–820.
19. Park JS, Son KR, Na DG, Kim E, Kim S. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. AJR Am J Roentgenol. 2009. 192:66–72.
20. Bournaud C, Charrie A, Nozieres C, Chikh K, Lapras V, Denier ML, et al. Thyroglobulin measurement in fine-needle aspirates of lymph nodes in patients with differentiated thyroid cancer: a simple definition of the threshold value, with emphasis on potential pitfalls of the method. Clin Chem Lab Med. 2010. 48:1171–1177.
21. Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981. 70:511–518.
22. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990. 71:414–424.
23. Noguchi S, Murakami N, Yamashita H, Toda M, Kawamoto H. Papillary thyroid carcinoma: modified radical neck dissection improves prognosis. Arch Surg. 1998. 133:276–280.
24. Roh JL, Park CI. Sentinel lymph node biopsy as guidance for central neck dissection in patients with papillary thyroid carcinoma. Cancer. 2008. 113:1527–1531.
25. Lee SK, Choi JH, Lim HI, Kim WW, Kim SM, Choe JH, et al. Sentinel lymph node biopsy in papillary thyroid cancer: comparison study of blue dye method and combined radioisotope and blue dye method in papillary thyroid cancer. Eur J Surg Oncol. 2009. 35:974–979.
26. Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998. 133:419–425.
27. Noguchi S, Noguchi A, Murakami N. Papillary carcinoma of the thyroid. I Developing pattern of metastasis. Cancer. 1970. 26:1053–1060.
28. Noguchi S, Murakami N. The value of lymph-node dissection in patients with differentiated thyroid cancer. Surg Clin North Am. 1987. 67:251–261.
29. Mirallie E, Visset J, Sagan C, Hamy A, Le Bodic MF, Paineau J. Localization of cervical node metastasis of papillary thyroid carcinoma. World J Surg. 1999. 23:970–973.
30. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. 2006. 30:91–99.




 ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download