Abstract
A gastric carcinoma with the endoscopic features resembling submucosal tumor (SMT) is rare, and reportedly account for only 0.1% to 0.63% of all resected gastric carcinomas. The preoperative diagnosis of SMT-like gastric carcinoma is challenging, and thus, diagnosis is usually made intraoperatively or postoperatively. Furthermore, mucinous adenocarcinoma is an uncommon histologic subtype of gastric carcinoma characterized as an elevated lesion resembling SMT due to abundant mucin pools in submucosa. Here, we report two cases in which a gastric mucinous adenocarcinoma was mistaken as a SMT.
Most gastric adenocarcinomas manifest mucosal lesions, and thus, they are usually diagnosed by endoscopic biopsy. In contrast, gastric submucosal tumor (SMT) is often difficult to diagnose histologically, because the tumor surface being covered with normal mucosa. A gastric carcinoma with the endoscopic features resembling SMT is rare, and reportedly account for only 0.1% to 0.63% of all resected gastric carcinomas in Japan [1]. A diagnosis of SMT-like gastric carcinoma is challenging as the tumors are entirely covered with normal mucosa. Furthermore mucinous gastric adenocarcinoma is uncommon histologic subtype of gastric cancer. Mucinous gastric adenocarcinoma is characterized as an elevated lesion resembling SMT due to abundant mucin pools in the submucosa. We encountered two cases of a mucinous gastric adenocarcinoma was mistaken for a gastric SMT, and here, we provide descriptions of these cases.
A 54-year-old woman underwent endoscopy at local clinic for symptoms of dyspepsia, vomiting, and poor oral intake. Because endoscopic findings suggested a gastric SMT, the patient was referred to our hospital. The biopsy sample obtained from the lesion at the other clinic was not diagnosed histologically as malignant, and repeat endoscopy at our hospital showed two round, large protruding masses in the antrum with partial obstruction, but without a mucosal abnormality (Fig. 1A). Endoscopic ultrasonography (EUS) revealed two large cystic lesions with nonhomogenous mixed echoic masses in the proper muscle layer, without nearby lymph node enlargement (Fig. 1B). Computed tomography (CT) also depicted the lesions observed by EUS (Fig. 1C). No repeat biopsy was taken. Based on these findings, a gastric SMT was diagnosed, and laparoscopy-assisted antrectomy without lymph node dissection was performed. A protuberant lesions, 4.5 × 3.5 cm in size, was found in the antrum. Microscopic examination revealed the cancer tissue was predominantly located in the submucosal and proper muscle layers (Fig. 2B). The pathologic diagnosis was mucinous adenocarcinoma and the lesion was found to have invaded the subserosa, and no lymph node was excised (Fig. 2C). Thus, the disease was stages as T3Nx. Although additional surgery was recommended, this was rejected by the patient, and no follow-up was made.
A 50-year-old female patient was found to have a gastric tumor during a screening examination performed at another hospital and was referred to our hospital. She had no particular or family history of gastric disease and did not manifest any digestive system related symptom. Endoscopy performed at the other hospital showed an extrinsic mass effect in the lesser curvature of the midbody without mucosal lesion (Fig. 3A). Endoscopic biopsy on the area where the mass was observed suggested chronic active gastritis.
CT showed a well-circumscribed, exophytic, submucosal 5 cm tumor in the lesser curvature of the midbody, partial necrosis inside the mass, and lymph node enlargement around the lesion (Fig. 3B). Based on these findings, malignant gastrointestinal stromal tumor was suspected, and laparoscopic partial gastrectomy was planned without a repeat endoscopic examination.
The surgical findings revealed numerous lymph node enlargements around the mass and severe adhesion between the posterior wall of the stomach and surrounding tissues. Thus, a frozen section of a lymph node was first examined, and then laparotomy was performed. Frozen section examination suggested metastatic adenocarcinoma, and thus, we decided that surgery for advanced gastric cancer was required. Radical total gastrectomy with D2 lymph node dissection was undertaken and there was a round bulging of the mass in the lesser curvature of midbody and the mucosal surface is slightly red in comparison with the surrounding gastric mucosa but no erosion is evident (Fig. 4A), and gross findings showed tumor infiltration of subserosa (Fig. 4B).
The histopathological diagnosis was mucinous adenocarcinoma and there was no mucosal involvement of the carcinoma microscopically (Fig. 4C). The carcinoma was found to have invaded the serosa and metastasized to three of the 34 excised lymph nodes, and thus, the cancer was staged as IIIB (T4aN2M0). The patient was discharged without any particular complication and is currently on outpatient follow-up.
Gastric adenocarcinoma without a mucosal lesion is rare, and has a prevalence of only 0.2% to 0.6% [1]. It is considered that excessive infiltration of lymphocytes in gastric cancer, intensive secretion of mucus by mucous adenocarcinoma, adenocarcinoma in the submucosal ectopic pancreas, and excessive fibrosis around gastric cancer may be responsible for gastric adenocarcinoma without mucosal lesion [1,2]. The majority of gastric adenocarcinomas without mucosal lesions occur in the upper and middle third of the stomach and are accompanied by central depression of mucosa and invasion of the muscular layer [2]. However, in our first case studied, the cancer originated from the antrum and infiltrated subserosa, whereas in the second case, the cancer was advanced and had originated from the midbody and infiltrated serosa without causing a change in mucosa.
The preoperative diagnosis of submucosal tumors resembling gastric adenocarcinoma is challenging, and thus, a diagnosis of adenocarcinoma is usually made intraoperatively or postoperatively. In fact, a review of the literature revealed that about 30% of such cancers are not diagnosed by endoscopic biopsy prior to surgical resection [2]. For preoperative diagnosis, it is important to observe findings, such as, amorphous rubor, erosion, depression, and a marginal white coating with clear boundaries at one side of the tumor during gastroscopy [2]. SMTs resembling gastric cancer differ from SMTs as follows: 1) they have a low elevation and an amorphous basal area; 2) a wide area of depression in the entire elevated area; and 3) an amorphous depression, and furthermore, may show incurvation [3]. Unfortunately, we did not take repeat endoscopy and biopsy, so no diagnosis was made preoperatively.
Although we did not check carbohydrate antigen 19-9 (CA 19-9) levels in either case, it has been reported that an elevated CA 19-9 level is associated with the progress and prognosis of stomach cancer, especially of mucinous adenocarcinoma [4]. Accordingly, knowledge of the preoperative CA 19-9 level could aid diagnosis. Endoscopic ultrasound of a submucosal tumor resembling gastric cancer, mucinous tumors appear hyperechoic with clear boundaries in the third layer, and SMTs show a continuous, clear, homogenous hypoechoic area in the fourth layer of the gastric wall. In our first case, EUS showed a hyperechoic tumor with clear boundaries in the third layer. However, despite this finding making an accurate diagnosis was challenging, and thus, EUS-guided biopsy involving mucosal incision and diagnostic endoscopic mucosal resection is currently recommended [5].
The differential diagnosis of submucosal tumor resembling gastric cancer and SMT is important because the two require different surgical treatments. For a small and typical SMT, outpatient follow-up can be performed in some cases, and even when surgery is performed, its goal is margin-free resection rather than radical gastrectomy [6,7]. Therefore, the postoperative diagnosis of gastric adenocarcinoma necessitates additional surgery and consequently burdens both patients and clinicians. As such, every effort should be made to achieve an accurate diagnosis before surgery. Furthermore, when gastric cancer is diagnosed intraoperatively or postoperatively, radical gastrectomy and lymphadenectomy are required, according to the principles of gastric-cancer surgery.
In conclusion, although diagnosing submucosal tumor resembling gastric cancer is difficult, it is important to make a concerted effort to do so, including repeated biopsy and EUS. In addition, if intraoperative findings suggestive of gastric cancer are found, it is important to determine the appropriate surgical scope for radical cure based on tests, such as, a frozen section examination.
Figures and Tables
 | Fig. 1(A) Endoscopy revealed two large round, protruding masses in the antrum without a mucosal abnormality. (B) Endoscopic ultrasonography showed two large cystic lesions containing nonhomogenous mixed echoic masses in the proper muscle layer, without nearby lymph node enlargement. (C) Computed tomography revealed a -3.1 cm sized multilocular cystic mass in the submucosal portion of the pylorus. |
 | Fig. 2(A) Cross section showing tumor infiltration into proper muscle and subserosa with normally appearing overlying mucosa. (B) The tumor was located under the intact mucosa (H&E, ×12.5). (C) The tumor showing focal papillary growth and extracelluar pools of mucin (H&E, ×100). |
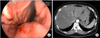 | Fig. 3(A) Endoscopy revealed an extrinsic mass effect in the lesser curvature of the midbody, and there was redness of mucosa around the lesion. However there was no evident erosion. (B) Computed tomography revealed a -5 cm sized mass with partial necrotic change in the lesser curvature side of the stomach body and multiple lymphadenopathy. |
 | Fig. 4(A) The mucosal surface is slightly red in comparison with the surrounding gastric mucosa but no erosion is evident. (B) On cross section, the tumor was found to have infiltrated subserosa. The tumor had a brownish tan color and necrotic change was observed in its center. (C) Low power histologic examination reveals most of the cancer cells are covered with noncancerous epithelium (H&E, ×12.5). |
References
1. Umehara Y, Kimura T, Okubo T, Sano Y, Nakai K, Oi S, et al. Gastric carcinoma resembling submucosal tumor. Gastric Cancer. 1999. 2:191–193.
2. Toyohiko Y, Tadasu S, Kazuhiko I, Shigeharu S, Satoshi S, Dai H, et al. Clinicopathological and imaging features of gastric carcinoma resembling submucosal tumor. Stomach Intest. 2003. 38:1527–1536.
3. Noriya U, Hiroyasy I, Shingo I, Hiroshi S, Shuji O, Tsutomo K, et al. Mucinous adenocarcinoma of the stomach mimicking submucosal tumor: report of a case. Stomach Intest. 2003. 38:1557–1561.
4. Nomura H, Mai M. Enhanced expression of CA19-9 in mucinous gastric carcinoma. Am J Gastroenterol. 1997. 92:2331–2332.
5. Teraishi F, Uno F, Kagawa S, Fujiwara T, Gouchi A, Tanaka N. Advanced gastric adenocarcinoma mimicking a submucosal tumor. Endoscopy. 2007. 39:Suppl 1. E191–E192.
6. Ohara N, Tominaga O, Uchiyama M, Nakano H. A case of advanced gastric cancer resembling submucosal tumor of the stomach. Jpn J Clin Oncol. 1997. 27:423–426.
7. Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002. 3:655–664.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download