Abstract
Purpose
Radiofrequency ablation (RFA) is a widely accepted to treat the varicose vein. However, outcome studies for occlusion rate and patterns of the saphenous vein after RFA are scarce. The purpose of our study is to report the results of RFA in patients with varicose vein.
Methods
We retrospectively reviewed the clinical outcomes after RFA using ClosureFAST (Covidien) catheter. We evaluated the occlusion rate and patterns with duplex scanning after RFA.
Results
A total of 200 limbs (148 patients) underwent RFA. The truncal veins were ablated in 163 great saphenous veins (GSV) and 41 small saphenous veins (SSVs). The mean age was 52.1 ± 11.9 years and female to male ratio was 125 : 87. At the mean follow-up of 13.9 months, the CEAP score, VCSS, and QoL score were significantly improved 2.33 ± 0.78 to 1.29 ± 0.96 (P < 0.0001), 3.48 ± 0.98 to 0.63 ± 1.16 (P < 0.0001), and 6.91 ± 6.69 to 3.38 ± 4.74 (P < 0.0001), respectively. The occlusion rate was 94.6% (53/56) in GSV and 94.5% (17/18) in SSV. The most common occlusion pattern in GSV was total occlusion of main trunk with patent superficial inferior epigastric vein in 41.1%. And, the most common pattern in SSV was the total occlusion of SSV with stump in 66.7%.
Varicose vein is common disease. The reported incidence of varicose vein ranges from 1% to 73% for females and 2% to 56% in males [1]. Before the endovenous era, high ligation and stripping of the saphenous vein was the standard treatment for the patients with varicose vein. Approximately 10 years ago, endovenous treatment of varicose veins such as radiofrequency ablation (RFA) and endovenous laser ablation (EVLA) became available. Recent randomized controlled trials revealed that endovenous treatment showed better results than traditional high ligation and stripping in terms of reduced pain, better quality of life (QoL), faster recovery, and lower rate of recurrence [2,3]. Because of the better clinical effects, the Society for Vascular Surgery and the American Venous Forum (AVF) recommended endovenous treatment of the incompetent saphenous vein over conventional high ligation and stripping [4].
Although the RFA and EVLA venous occlusion rates were comparable after treatment of primary varicose veins, RFA was associated with less periprocedural pain, analgesic requirement, and bruising [5,6]. However, long-term outcomes studies for the occlusion rate and patterns of the saphenous vein after RFA are scarce. The purpose of our study is to report the results of RFA in patients with varicose vein.
A retrospective review was performed in prospectively collected data of the patients who underwent surgical treatment of varicose vein from Mar. 2009 to Jun. 2011. We reviewed clinical outcomes of the patients who underwent RFA. On the initial encounter at outpatient office, we performed a history, physical examination, and duplex scan. A history included presence of symptoms related to varicose vein, risk factors, family history of varicose vein and a questionnaire about the QoL. The QoL score was evaluated by the Aberdeen Varicose Vein Questionnaire (AVVQ) assessment tools. The AVVQ is a validated tool for measurement of disease-specific QoL in patients with varicose veins. It produces a score from 0 (no venous symptom) to 100 (worst venous symptom) [7]. Clinical assessment according to CEAP classification was recorded at first visit. Clinical severity was assessed with Venous Clinical Severity Score (VCSS) revised by AVF Ad Hoc Outcomes Working Group in 2010 [8].
Initial imaging evaluation was made with the colorized duplex scan (Vivid E9 Ultrasound system, GE Healthcare, Waukesha, WI, USA). Saphenofemoral, saphenopopliteal, or truncal vein reflux in response to a Valsalva's maneuver and/or manual distal compression followed by release with upper body elevation or with standing position was identified with duplex scanning. The saphenous incompetence was defined by a reflux time more than 0.5 second on duplex scanning.
The indication for RFA was CEAP clinical class C2-C6 and patients with symptoms or cosmetic concern. The contraindication for RFA was deep vein insufficiency in the same extremity, the diameter of truncal vein ≥20 mm, meander and superficial truncal veins with a distance of <5 mm to the skin surface and the patients with recent cancer diagnosis. The RFA procedure was performed with general, spinal or tumescent anesthesia at the operating room in all patients. In our center, concomitant high ligation of the saphenous vein was not done. A phlebectomy around knee joint and below the knee joint was performed simultaneously. A ClosureFAST (Covidien, Mansfield, MA, USA) catheter was used for RFA. The RFA procedure was performed according to the manufacturer's instructions for use. The treatment consisted of segmental heating of the great saphenous vein (GSV), small saphenous vein (SSV) or accessory saphenous vein using a catheter with a 7-cm heating element. The catheter was advanced to 2 cm below the saphenofemoral or saphenopopliteal junction under ultrasound guidance. About 10 mL of tumescent solution per one centimeter of truncal vein was injected around the vein before the start of ablation. The temperature was maintained at 120° for 20 seconds per segment using a thermocouple on the heating element, which provided a feedback loop to the generator during withdrawal. Double treatment of the most proximal segment was performed. If the incompetent perforating vein was present at the ablated segment, double treatment was done. External compression of the treated segment was applied using a towel.
At the completion of procedure, all wounds were dressed with steri-strips and legs were placed in full-length Cohesive elastic bandage (Karl Otto Braun GmbH & Co, Wolfstein, Germany). The bandage was exchanged for thigh-length compression stocking after 24 hours. This was then worn for a minimum of 2 weeks. Patients were discharged on the postoperative day one with a stocking. They were advised to use analgesics only when required and return to work when they felt it was appropriate.
Patients were followed up at 1 week and 6 months after surgery. At all subsequent visits, the patients were examined clinically and with duplex scanning. At 1 week, duplex scanning was performed to confirm saphenous vein occlusion and to evaluate any complications such as deep vein thrombosis, hematoma, endovenous heat-induced thrombosis (EHIT), or any complications related with surgery. At 6 months, a further duplex scan was performed to evaluate any complications. Another clinical examination and duplex scanning was performed at 2 years after the first RFA in our institute. Clinical assessment and severity score were obtained according to CEAP classification and VCSS, respectively. The technical result and complications were recorded, and patients completed the QoL score with AVVQ. Duplex scanning was performed to evaluate the occlusion or recanalization, the occlusion pattern and to measure the length between the saphenofemoral or saphenopopliteal junction to the leading occlusion point of saphenous vein. Fig. 1 showed how to measure the length between the saphenous junction and leading point of occlusion. The gray-scale image was used in most patients. If the measurement was difficulty due to deep position of vessel, color Doppler image or power Doppler image were used.
The paired and independent t-test using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis to evaluate the clinical improvement after treatment. Null hypotheses of no difference were rejected if P-values were less than 0.05.
Between March 2009 to Junuary 2011, 212 consecutive patients (285 legs) underwent varicose vein treatment. Patient characteristics are summarized (Table 1). The mean age was 52.1 ± 11.9 years, median age 53 years, a range of 19 to 78 years. The female to male ratio was 125 : 87. The most common clinical score of CEAP classification was C2. There were 4 patients with active venous ulcers around the ankle. During the period, RFA was performed to 148 patients. The other types of surgery such as high ligation & stripping, perforator ligation only, and EVLA were performed in 59 patients, 4 patients and 1 patient, respectively. According to our institute's policy, RFA was primarily performed to the patients with varicose vein unless there was any contraindication for RFA.
A total of 200 limbs in 148 patients underwent RFA. All saphenous veins with reflux ≥0.5 second were ablated simultaneously. With one session, one truncal vein was ablated in 96 patients, 2 truncal veins in 49 patients, and 3 truncal veins in 2 patients. Four truncal veins were ablated simultaneously in one patient. The truncal veins were ablated in 163 GSVs and 41 SSVs. If the patient had the refluxed GSV and SSV, RFA was performed in supine position. The patient was placed with prone position if the refluxed vein was unilateral or bilateral SSV. After RFA, there were several types of complication (Table 2). The most common complication was paresthesia, which occurred in 12 patients (8.1%). Another complication was the palpation of cord-like mass at the site of ablation in 5 patients (3.4%). The most difficult complication was deep vein thrombosis or thrombus extension into the common femoral vein. In our series, two patients (1.3%) had thrombus extension into the common femoral vein. A classification of EHIT has been developed to grade the extent of the thrombus and to correlate ultrasound findings with a treatment plan on 2006 [9]. All two patients in our series had a class II EHIT. We did not use any anticoagulation to treat the EHIT.
The clinical outcomes are summarized in Table 3. A total of 52 patients were followed up. The mean follow-up period was 13.9 ± 6.9 months. All parameters of clinical outcomes were significantly improved by the paired t-test. The mean preoperative score of CEAP clinical classification was 2.33 ± 0.78 and postoperative mean score was 1.29 ± 0.96. The difference of preoperative and postoperative CEAP clinical score was 1.03 ± 0.43 (P < 0.0001). The analysis for the difference of VCSS showed significantly improved (P < 0.0001). We analyzed the difference of QoL score with AVVQ score. Preoperative score was 6.91 ± 6.69 and postoperative score was 3.38 ± 4.74. The difference of QoL score was 3.53 ± 2.57 (P < 0.0001).
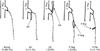
After 2 years from first RFA, the occlusion rate of the truncal vein was evaluated with duplex scanning in 74 limbs of 52 patients. The GSV was evaluated in 56 limbs and SSV was evaluated in 18 limbs. The occlusion rates were 94.6% (53/56) in GSV and 94.5% (17/18) in SSV. The partially occluded GSV was found in 3 patients. The partial occlusion of GSV was seen at one week, 26 months, and 28 months after RFA, respectively. The partial occlusion of SSV was seen in one patient at 9 months after RFA. According to the Recurrent Varices after Surgery criteria proposed by Perrin et al. [10], there was no clinically significant reflux or source of reflux and topographical sites were thigh. It was only a sonographic finding. There was one patient with the partially occluded SSV. The patient had not symptomatic recurrence, but only a sonographic finding. The occlusion rate between GSV and SSV was similar (Fig. 2). We measured the length between the saphenofemoral junction to leading point of occlusion in GSV and between the saphenopopliteal junction to its in SSV. In GSV, the mean length was 11.6 ± 8.7 mm in GSV and 11.5 ± 10.2 mm in SSV (P = 0.142 by independent t-test). The occlusion patterns of GSV (Fig. 3) and SSV (Fig. 4) were evaluated with duplex scanning 2 years after initial RFA. We could classify the 6 types of occlusion pattern in GSV. We defined total occlusion if the main truncal vein was occluded in whole ablated segment. The most common pattern was total occlusion of GSV with patent superficial inferior epigastric vein in 23 limbs (41.1%). There were 5 types of occlusion patterns in SSV. The most common pattern was total occlusion of SSV with short segment of stump in 12/18 (66.7%).
There are several reports evaluating the occlusion rate of saphenous vein after RFA. Helmy et al. [11] evaluated the occlusion rate in a total of 90 patients up to 24 months following RFA. The primary occlusion rate was 94.5%. Proebstle et al. [12] observed occlusion in 256 of 295 treated GSVs (86.4%) with a prospective multicenter trial. At 36 months, Kaplan-Meier survival analysis showed the probability of occlusion was 92.6% and the probability of no reflux was 95.7%, and 96.9% of legs remained free of clinically relevant axial reflux. In our series, the occlusion rate was 94.6% (53/56) in GSV and 94.5% (17/18) in SSV. However, some workers reported lower occlusion rates after RFA. Merchant et al. [9] reported the data of a prospective registry for 286 patients from 30 clinical sites. The occlusion rate was 83.6% at 12 months and 85.2% at 24 months, respectively. There are three possible causes of the difference for occlusion rates between the studies: ablation temperature, mechanism of heat transmission, and differences in surgical technique. The ablation temperature of ClosurePlus catheter used in Merchant's study is 85 ± 2℃, but the temperature of the ClosureFast catheter used in former two studies including our series is 120℃. The mechanism of heat transmission is different between the devices. ClosurePlus catheter has multiple pods whose portions only can transmit heat. But heat is transmitted evenly in all directions in a ClosureFast catheter.
In our study, the occlusion patterns of GSV were categorized into six types. The most common type was complete occlusion (CO) of GSV with patent inferior epigastric vein. Merchant et al. [9] classified the occlusion pattern into three types: CO veins were defined as those with no evidence of flow. Near complete occlusion (NCO) was defined as less than or equal to 5-cm segment of flow within an otherwise occluded vein. Recanalization was defined as greater than 5 cm of flow in any treated vein segment. At 24 months, 85.2% of treated veins were CO, 3.5% were NCO, and 11.3% were recanalized. In our study, 94.6% of treated veins were CO, 2.7% were NCO, and 2.7% were recanalized.
There has been no prior report of the occlusion patterns of the SSV after RFA to our knowledge. We found five distinct types. The most common type was occlusion of SSV with patent short segment of proximal portion. In our study, we could perform follow-up duplex scan in only 18 patients to evaluate the occlusion pattern of SSV. The confluence of SSV in the deep system proved to be highly variable. The SSV usually terminates at the diamond shaped popliteal space, joining the popliteal vein directly or via the gastrocnemius vein. According to the Labropoulos's study, SSV joined the popliteal vein just above the popliteal crease in 57.8% of the limbs, whereas the SSV terminated in the thigh in 26.6% [13]. Another anatomic variation of the SSV was reported. Delis et al. [14] reported the anatomic patterns of the SSV with duplex examination. A Giacomini vein was found in 70.4% of limbs. With these anatomic variations of SSV, more occlusion patterns will be found after RFA.
One of the recognized complications is extension of thrombus from the saphenous vein into the deep venous system following RFA. It is a thrombus extending from the superficial venous system into the deep venous system at, or proximal to, a site of recent thermoablation. It is described with the classification system of EHIT with four classes [15]. Reported rates of EHIT vary widely from 0% to 16% post-RFA and 0 to 7.7% post-EVLA [16]. In our series, two patients (1.3%) had thrombus extension into the common femoral vein. Because of this complication, the routine postoperative DUS is warranted [15]. Currently, there is no evidence-based recommendation for EHIT management. Lawrence et al. [17] developed a six-tier classification system based on thrombus proximity to the epigastric or femoral vein, and an algorithm for treatment. They recommended the use of low molecular weight heparin (LMWH) if the thrombus protruded into deep vein. Marsh et al. [15] recommended the anticoagulation with LMWH in patients with EHIT until resolution. Other managements of EHIT were reported operative thrombectomy, anticoagulation, saphenofemoral ligation, and caval filter insertion with thrombolysis [18,19]. Our study has several limitations. Data were collected retrospectively and the followed-up patients were relatively small.
In conclusion, RFA is an effective modality in the treatment of varicose vein. The clinical parameters including CEAP class, VCSS, QoL score showed the significant improvement after RFA. At the mean follow-up of 13.9 months, the occlusion rate was 94.6% in GSV and 94.5% in SSV. The most common occlusion pattern was total occlusion of GSV with patent superficial inferior epigastric vein in GSV and total occlusion of SSV with small stump in SSV.
Figures and Tables
Fig. 1
The measurement between the saphenous junction and leading point of occlusion. (A) Twelve months after radiofrequency ablation of great saphenous vein: The distance between the saphenous junction and leading point of occlusion was 1.63 cm. (B) Six months after ablation of small saphenous vein: a color Doppler image was used for measurement due to relatively deep position of vein. The distance was measured as 0.802 cm.
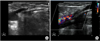
Fig. 2
Occlusion rate of saphenous vein. Kaplan-Meier analysis showed the similar occlusion rate between the great saphenous vein and small saphenous vein. GSV, great saphenous vein; SSV, small saphenous vein.

Fig. 3
Occlusion patterns of the great saphenous vein. This diagram showed 6 types of occlusion pattern of the great saphenous vein (GSV) after radiofrequency ablation. The most common type was total occlusion of GSV with patent inferior epigastric vein. CFV, common femoral vein; Epi, inferior epigastric vein; AL Br, anterolateral branch of GSV; P Br, patent branch; Br, branch.

Fig. 4
Occlusion patterns of the small saphenous vein. This diagram showed 5 types of occlusion pattern of the small saphenous vein (SSV) after radiofrequency ablation. The most common type was total occlusion of SSV with short patent stump. Pop. V, popliteal vein; SV, sural vein; CE, cranial extension of SSV; P Seg, patent segment; P Per, patent perforating vein.
References
1. Lohr J, Kulwicki A. Radiofrequency ablation: evolution of a treatment. Semin Vasc Surg. 2010. 23:90–100.
2. Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg. 2011. 98:501–510.
3. Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011. 98:1079–1087.
4. Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011. 53:5 Suppl. 2S–48S.
5. Goode SD, Chowdhury A, Crockett M, Beech A, Simpson R, Richards T, et al. Laser and radiofrequency ablation study (LARA study): a randomised study comparing radiofrequency ablation and endovenous laser ablation (810 nm). Eur J Vasc Endovasc Surg. 2010. 40:246–253.
6. Nordon IM, Hinchliffe RJ, Brar R, Moxey P, Black SA, Thompson MM, et al. A prospective double-blind randomized controlled trial of radiofrequency versus laser treatment of the great saphenous vein in patients with varicose veins. Ann Surg. 2011. 254:876–881.
7. Garratt AM, Macdonald LM, Ruta DA, Russell IT, Buckingham JK, Krukowski ZH. Towards measurement of outcome for patients with varicose veins. Qual Health Care. 1993. 2:5–10.
8. Vasquez MA, Rabe E, McLafferty RB, Shortell CK, Marston WA, Gillespie D, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010. 52:1387–1396.
9. Merchant RF, DePalma RG, Kabnick LS. Endovascular obliteration of saphenous reflux: a multicenter study. J Vasc Surg. 2002. 35:1190–1196.
10. Perrin MR, Guex JJ, Ruckley CV, dePalma RG, Royle JP, Eklof B, et al. REVAS group. Recurrent varices after surgery (REVAS), a consensus document. Cardiovasc Surg. 2000. 8:233–245.
11. Helmy ElKaffas K, ElKashef O, ElBaz W. Great saphenous vein radiofrequency ablation versus standard stripping in the management of primary varicose veins-a randomized clinical trial. Angiology. 2011. 62:49–54.
12. Proebstle TM, Alm J, Gockeritz O, Wenzel C, Noppeney T, Lebard C, et al. Three-year European follow-up of endovenous radiofrequency-powered segmental thermal ablation of the great saphenous vein with or without treatment of calf varicosities. J Vasc Surg. 2011. 54:146–152.
13. Labropoulos N, Leon M, Nicolaides AN, Giannoukas AD, Volteas N, Chan P. Superficial venous insufficiency: correlation of anatomic extent of reflux with clinical symptoms and signs. J Vasc Surg. 1994. 20:953–958.
14. Delis KT, Knaggs AL, Khodabakhsh P. Prevalence, anatomic patterns, valvular competence, and clinical significance of the Giacomini vein. J Vasc Surg. 2004. 40:1174–1183.
15. Marsh P, Price BA, Holdstock J, Harrison C, Whiteley MS. Deep vein thrombosis (DVT) after venous thermoablation techniques: rates of endovenous heat-induced thrombosis (EHIT) and classical DVT after radiofrequency and endovenous laser ablation in a single centre. Eur J Vasc Endovasc Surg. 2010. 40:521–527.
16. Mozes G, Kalra M, Carmo M, Swenson L, Gloviczki P. Extension of saphenous thrombus into the femoral vein: a potential complication of new endovenous ablation techniques. J Vasc Surg. 2005. 41:130–135.
17. Lawrence PF, Chandra A, Wu M, Rigberg D, DeRubertis B, Gelabert H, et al. Classification of proximal endovenous closure levels and treatment algorithm. J Vasc Surg. 2010. 52:388–393.
18. Manfrini S, Gasbarro V, Danielsson G, Norgren L, Chandler JG, Lennox AF, et al. Endovenous Reflux Management Study Group. Endovenous management of saphenous vein reflux. J Vasc Surg. 2000. 32:330–342.
19. Hingorani AP, Ascher E, Markevich N, Schutzer RW, Kallakuri S, Hou A, et al. Deep venous thrombosis after radiofrequency ablation of greater saphenous vein: a word of caution. J Vasc Surg. 2004. 40:500–504.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download