Abstract
Purpose
The reflux of pancreatic enzymes into the biliary tract is associated with chronic inflammation and increases cellular proliferation of the biliary epithelium, leading to biliary carcinoma. The aim of this study is to detect the incidence of occult pancreaticobiliary reflux (OPBR) in patients who underwent elective cholecystectomy.
Methods
Forty-seven patients with symptomatic gallstones who underwent cholecystectomy were recruited for this study. The gallbladder bile samples were obtained from the specimen of gallbladder and the amylase level was measured. The immunohistochemistry of p53, SMAD4 and Ki-67 were performed for the detection of metaplasia and dysplasia.
Results
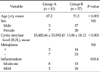
Biliary amylase was higher than the serum amylase in 10 patients (group A, 15,402.66 ± 33,592.43 IU/L; group B, 13.06 ± 18.12 IU/L). The mean age was 67.2 years in group A and 51.2 in group B (P < 0.01). The ratio of male to female was 1:2.3 and 1:1.8 in group A and B, respectively (P = 0.297). Eight patients in group A and thirteen patients in group B had inflammation (P = 0.014). The positive results of the Ki-67 test were exhibited in five cases in each group (P = 0.024).
Conclusion
Results from the study indicate that the age was older, degree of inflammation and positive rate of Ki-67 were higher when OPBR was suspected. In conclusion, the patients with OPBR would need long-term follow-up, because the OPBR can cause dysplasia and the reflux of pancreatic juice may be considered as a risk factor for extrahepatic bile duct carcinoma.
The reflux of pancreatic juice into the biliary tract usually occurs in patients with anomalous pancreaticobiliary ductal union (APBDU), and the regurged pancreatic juice in the bile duct due to APBDU is known as an important risk factor for extrahepatic bile duct carcinoma. In particular, the mixture of regurged pancreatic juice and bile in the gallbladder is associated with a high incidence of gallbladder carcinoma [1]. However, the high level of biliary amylase can occur in patients with an anatomically normal pancreaticobiliary junction, defined as the occult pancreaticobiliary reflux (OPBR), and this can be associated with biliary malignancy as in the case of APBDU [2-5]. High levels of biliary amylase in a morphologically normal pancreaticobiliary duct can occur due to pressure differences between the sphincter of Oddi and the duodenum. The spastic sphincter of Oddi plus the lack of sphincter function is associated with reflux of pancreatic juice [6,7]. Although it is important to evaluate the presence of APBDU by endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholagniopancreatograpy (MRCP) to confirm the OPBR, there are some difficulties of the performance of these expensive and invasive image modalities in patients with benign gallbladder disease. Although Beltran et al. [4] and Itokawa et al. [3] reported that the incidence of OPBR was approximately 84.2% and 25%, respectively, there have been limited studies regarding OPBR. Authors measured the amylase levels of bile in patients without bile duct dilatation who underwent laparoscopic cholecystectomy. We also evaluated the relationship between clinical and pathological characteristics.
From December 2010 to March 2011, 47 patients with symptomatic gallstones who underwent elective laparoscopic cholecystectomy were enrolled in this study. The mean operation time was 99.25 ± 10.38 minutes. Bile samples were obtained from the specimens of resected gallbladders within 30 minutes and the amylase level in the bile was measured. Patients with preoperative ERCP and PTGBD were excluded and patients with gallstone pancreatitis and acute cholecystitis were excluded. Patients were categorized into group A, those who had higher levels of amylase in the bile acid than in the blood serum and group B, those who did not. In the group A, patients with APBUU on the preoperative CT scan were excluded.
The normal range for serum amylase is 36-128 IU/L and high biliary amylase was defined as a biliary amylase level above 128 IU/L.
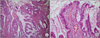
We classified the grade of inflammation from mild to severe according to the degree of infiltrating inflammatory cells. When the infiltrated inflammatory cells were present mostly in the lamina propria of the mucosa, it was diagnosed as mild inflammation. When the infiltrating inflammatory cells were mostly detected in the muscle layer and beyond the muscle layer, moderate to severe inflammation was diagnosed, respectively (Fig. 1). The metaplasia, which is a common manifestation of chronic injury to the biliary duct, was divided into the pyloric gland type and intestinal type (Fig. 2). The immunohistochemistry of p53, SMAD4 and Ki-67 were performed (Fig. 3).
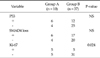
The mean age was 67.2 years old in group A and 51.2 years old in group B. There were significant differences towards older patients in group A (P < 0.001). The ratio of gender was 1:2.3 in group A and 1:1.8 in group B (P = 0.297). The mean amylase level in the bile was 15,402.66 ± 33,592.43 IU/L and 13.06 ± 18.12 IU/L in group A and group B, respectively. The metaplasia was found in three cases in group A and 17 cases in group B (P = 0.474). Eight patients had moderate inflammation in group A, which was significant compared to group B (P = 0.014) (Table 1). In the immunohistochemical staining, six cases in group A and 12 cases in group B showed positive results for the p53 analysis. The loss of SMAD4 was detected in six cases of group A and five cases of group B. However, there were no statistically significant differences in p53 and loss of SMAD4 analysis (P = 0.111, 0.333). On the other hand, the Ki-67 index, which is usually substantially greater in dysplastic lesions and increases in quantity with increasing degrees of dysplasia, was positive in five cases of each group with significance (P = 0.024) (Table 2).
The cellular proliferation of the biliary epithelium is facilitated when the regurgitation of pancreatic juice into the bile duct has occurred in APBUD, because of genetic alterations in the bile duct. The biliary tract can be changed into a hyperplastic or dysplastic state due to repetitive stimulation of regurged pancreatic juice onto the bile duct mucosa, and these changes can cause cancerous changes in the mucosa [8,9]. Based on previous studies, these changes in the biliary mucosa can also occur in the patient with OPBR [2-5,10-14]. The incidence of APBUD in the patients with biliary disorders who performed ERCP was reported as 1.5 % in Japan, 8.7 % in Taiwan and 1 % in Europe [9]. Thus, the incidence of OPBR would be much higher than APBDU. However, there are limited reports which describe the pancreaticobiliary reflux in bile in a large series of patients with OPBR. Although, to confirm the presence of APBDU, the MRCP, ERCP or intraoperative cholangiography is necessary, these imaging modalities are not cost-effective for patients with gallbladder stones only. However, measuring the amylase level in bile is a relatively easy method to detect the presence of APBUD or OPBR. Sakamoto et al. [5] described that the cutoff value for high cystic amylase was defined as a biliary amylase level higher than the normal upper limit of serum amylase. The authors measured the level of amylase of bile in patients who underwent laparoscopic cholecystectomy without any additional imaging to confirm anatomical abnormalities in the biliary tract. The OPBR was assumed when the level of biliary amylase was higher than the serum amylase, and 10 patients (21.3%) were suspected of OPBR among the 47 patients. In our study, the prevalence of OPBR was lower than the previous report of Beltran et al. [4], but, was similar to results reported by Itokawa et al. [3]. According to the previous studies, the age is not associated with OPBR, however, there was a significant difference toward older patients in the present study. The degree of inflammation was histopathologically more severe in the patients suspected of OPBR. Chronic inflammation by gallbladder stones in addition to pancreatic juice including amylase might play a pivotal role for the development of intestinal metaplasia in the gallbladder [15]. In the present study, the metaplasia was observed in both groups. The pyloric gland metaplasia was shown in most cases and with intestinal metaplasia in several cases. Dysplasia on the gallbladder mucosa could not be confirmed by histopathologic examination. However, the positive results with Ki-67 were significantly higher in the patients with high amylase levels in the immunohistochemical staining which was performed to identify the proliferation in the mucosa [10,12]. The Ki-67 labeling index is usually substantially greater in dysplastic lesions and increases in quantity with increasing degrees of dysplasia. It can also be marked in areas of regenerative change as well [8,12]. We found that high amylase level in the gallbladder was an important risk factor which causes inflammation and degeneration of the gallbladder mucosa. The positive results for the immunohistochemical staining of p53 are rarely detected in extrahepatic bile duct tumors. It is common in flat dysplasia and invasive carcinoma and positive in more than 30% of dysplastic lesions. However, a significant difference was not observed between dysplasia and normal mucosa [15]. SMAD4 is part of the transforming growth factor-Beta signaling pathway, where it plays a role in growth expression. Deletion and loss of SMAD4 expression occurs in about 50% of pancreatic cancers, and 30 to 50% of colorectal cancers. SMAD4 alterations occur in 24% of nonampullary small intestinal adenocarcinomas and 34% of ampullary carcinomas. Hahn et al. [16] demonstrated that DPC4/SMAD4 is indeed involved in the tumor formation of biliary tract cancers. However, these factors were not associated with OPBR in our study. There is still controversy regarding treatment of the patients with OPBR. However, the prophylactic cholecystectomy would be an effective treatment to reduce the risk of gallbladder cancer [17-19]. The incidence of bile duct cancer is higher in the patients with APBDU without biliary dilatation compared to patients with bile duct dilation. Similarly, the patients who have OPBR with biliary dilatation would have a higher risk of cancer compared to those who do not. Funabiki et al. [9] reported that incidence of bile duct cancer associated to APBDU without biliary dilatation is 4.0% in the last 10 years, and 5.2% in the patients with biliary dilatation. However, there was no significant difference in both groups. Of course, the risk of gallbladder cancer in the patients with APBDU or OPBR would be excluded after cholecystectomy, but physicians should remember that the risk of bile duct carcinoma still exists. To confirm APBDU, the MRCP or ERCP can be performed before the operation and intraoperative cholangiography may be performed during the operation. However, these diagnostic modalities have limitations with regards to cost-effectiveness for patients who will undergo laparoscopic cholecystectomy with benign gallbladder disease. Thus, relatively easy and cost-effective methods such as measuring the level of amylase in bile would be helpful to patients with OPBR.
In conclusion, the patient with OPBR would need long-term follow-up, because the reflux of pancreatic juice may be considered as a risk factor for extrahepatic bile duct carcinoma.
Figures and Tables
Fig. 1
(A) Mild inflammation of the gallbladder (H&E, ×100). (B) Moderate inflammation of the gallbladder (H&E, ×100).

Fig. 2
(A) Pyloric gland metaplasia in the gallbladder (H&E, ×100). (B) Intestinal metaplasia in the gallbladder (H&E, ×100).
ACKNOWLEDGEMENTS
This work was supported by clinical research grant from Pusan National University Hospital 2012.
This study was supported by a grant (0920050) from the National R&D Program for Cancer Control, Ministry for Health, Welfare, and Family Affairs, Republic of Korea, The biospecimens for this study were provided by the Pusan National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
References
1. Hanada K, Itoh M, Fujii K, Tsuchida A, Hirata M, Ishimaru S, et al. Pathology and cellular kinetics of gallbladder with an anomalous junction of the pancreaticobiliary duct. Am J Gastroenterol. 1996. 91:1007–1011.
2. Sai JK, Suyama M, Kubokawa Y, Tadokoro H, Sato N, Maehara T, et al. Occult pancreatobiliary reflux in patients with a normal pancreaticobiliary junction. Gastrointest Endosc. 2003. 57:364–368.
3. Itokawa F, Itoi T, Nakamura K, Sofuni A, Kakimi K, Moriyasu F, et al. Assessment of occult pancreatobiliary reflux in patients with pancreaticobiliary disease by ERCP. J Gastroenterol. 2004. 39:988–994.
4. Beltran MA, Vracko J, Cumsille MA, Cruces KS, Almonacid J, Danilova T. Occult pancreaticobiliary reflux in gallbladder cancer and benign gallbladder diseases. J Surg Oncol. 2007. 96:26–31.
5. Sakamoto H, Mutoh H, Ido K, Satoh S, Kumagai M, Hayakawa H, et al. Intestinal metaplasia in gallbladder correlates with high amylase levels in bile in patients with a morphologically normal pancreaticobiliary duct. Hum Pathol. 2009. 40:1762–1767.
6. Schweizer P, Schweizer M. Pancreaticobiliary long common channel syndrome and congenital anomalous dilatation of the choledochal duct: study of 46 patients. Eur J Pediatr Surg. 1993. 3:15–21.
7. Imazu M, Iwai N, Tokiwa K, Shimotake T, Kimura O, Ono S. Factors of biliary carcinogenesis in choledochal cysts. Eur J Pediatr Surg. 2001. 11:24–27.
8. Tanno S, Obara T, Fujii T, Mizukami Y, Shudo R, Nishino N, et al. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gallbladder in patients with anomalous pancreaticobiliary ductal union. Cancer. 1998. 83:267–275.
9. Funabiki T, Matsubara T, Miyakawa S, Ishihara S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009. 394:159–169.
10. Sai JK, Suyama M, Nobukawa B, Kubokawa Y, Sato N. Severe dysplasia of the gallbladder associated with occult pancreatobiliary reflux. J Gastroenterol. 2005. 40:756–760.
11. Vracko J, Markovic S, Wiechel KL. Conservative treatment versus endoscopic sphincterotomy in the initial management of acute cholecystitis in elderly patients at high surgical risk. Endoscopy. 2006. 38:773–778.
12. Sai JK, Suyama M, Nobukawa B, Kubokawa Y, Yokomizo K, Sato N. Precancerous mucosal changes in the gallbladder of patients with occult pancreatobiliary reflux. Gastrointest Endosc. 2005. 61:264–268.
13. Vracko J, Wiechel KL. Increased gallbladder trypsin in acute cholecystitis indicates functional disorder in the sphincter of oddi and could make EPT a logical procedure. Surg Laparosc Endosc Percutan Tech. 2003. 13:308–313.
14. Vracko J, Zemva Z, Pegan V, Wiechel KL. Sphincter of Oddi function studied by radioimmunoassay of biliary trypsin in patients with bile duct stones and in controls. Surg Endosc. 1994. 8:389–392.
15. Chiba H, Nagai H, Ohdaira T, Yasuda Y, Saito K. Immunohistochemical study of characteristics of bile duct dysplasia evaluated on the basis of expression of metastasis/invasion-related factors and p53. J Hepatobiliary Pancreat Surg. 2004. 11:409–416.
16. Hahn SA, Bartsch D, Schroers A, Galehdari H, Becker M, Ramaswamy A, et al. Mutations of the DPC4/Smad4 gene in biliary tract carcinoma. Cancer Res. 1998. 58:1124–1126.
17. Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, et al. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003. 10:345–351.
18. Ohuchida J, Chijiiwa K, Hiyoshi M, Kobayashi K, Konomi H, Tanaka M. Long-term results of treatment for pancreaticobiliary maljunction without bile duct dilatation. Arch Surg. 2006. 141:1066–1070.
19. Ichikawa Y, Kamiyama M, Sekido H, Ishikawa T, Miura Y, Kamiya N, et al. Telomerase activity and Bcl-2 expression in gallbladders of pancreaticobiliary maljunction patients: a preliminary study. J Hepatobiliary Pancreat Surg. 2004. 11:34–39.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download