Abstract
Colorectal cancer is the third most common malignancy in Korea. In contrast, pericolic or mesenteric lymphoma is relatively rare. We experienced an extremely rare case of synchronous primary colon cancer in the ascending colon with T-cell lymphoma in the pericolic lymph node. A 79-year-old woman presented with complaints of epigastric and right lower abdominal pain combined with anorexia and nausea. Colonoscopic evaluation and biopsy were performed, and the diagnosis was cecal adenocarcinoma. She underwent right hemicolectomy with lymph node dissection. The pathology report revealed adenocarcinoma in cecum with metastasis to 1 regional lymph node out of 37 lymph nodes. In addition, there was malignant angioimmunoblastic T-cell lymphoma in 1 pericolic lymph node. There was no evidence of lymphoma in ileum, cecum and ascending colon, so the possibility of early phase of lymphoma was suggested.
Colorectal cancer is the third most common malignancy in Korea [1]. In contrast, pericolic or mesenteric lymphoma is relatively rare. In addition, synchronous colonic carcinoma and lymphoma in the same patient is a rare occurrence. Probability was estimated at 0.0002% [2]. We report an extremely rare case of synchronous primary colon cancer in the ascending colon with T-cell lymphoma in the pericolic lymph node.
A 79-year-old woman presented with complaints of right side abdominal pain combined with anorexia, nausea and melena. Physical examination on admission revealed neither superficial lymphadenopathy nor hepatosplenomegaly. The solid and mobile mass was palpable at ileocecal region of the abdomen. Laboratory data showed hematocrit at 22.5%, hemoglobin at 6.6 g/dL, a white blood cell count of 12,300/mL (with a differential of neutrophils, 86.8%; lymphocytes, 8.7%; monocytes, 3.7%). Platelet count was 458,000/mL. Tumor marker was in normal range (carcinoembryonal antigen, 2.8 ng/mL; carbohydrate antigen 19-9, 9.0 U/mL). Her medical epidemiologic history was unremarkable.
Abdominal-pelvic computed tomography (CT) scan was performed, and colon cancer with pericolic lymph node metastasis was suggested (Fig. 1A, B). Cholecystitis due to gallbladder stone was also suggested. There was no metastatic lesion in the liver.
On colonoscopy, an ulcerofungating mass was seen in the cecum, which extended into the ileum (Fig. 2A, B). A colonoscopic biopsy was performed, and the diagnosis was adenocarcinoma with moderate to poor differentiation.
She underwent right hemicolectomy with lymph node dissection and cholecystectomy. Opening along the long axis showed an ulcerofungating tumorous lesion measuring 7 × 5 cm in the cecum. The mass was located 45 cm from proximal margin and 24 cm from distal margin (Fig. 3A). On section, the tumor infiltrated into the pericolic and mesenteric adipose tissue (Fig. 3B).
The pathology report of the resected cecal mass revealed usual colonic type adenocarcinoma with metastasis to 1 regional lymph node out of 37 lymph nodes. It was a moderately to poorly differentiated type. Grossly, lymphoreticular neoplastic lesion was not suspected in any of the pericolic lymph nodes (Fig. 4A). However, microscopically, one of the non-metastasized pericolic lymph nodes showed subtotal effacement (Fig. 4B). The lymphoid follicles are nearly absent showing "burned out" germinal center and follicular dendritic cell proliferation (Fig. 4C). The lymph node was replaced by atypical polymorphous lymphoid cells as with reactive plasma cells and eosinophils (Fig. 4D). Arborizing blood vessels corresponding to high endothelial venules were present. Additional immunohistochemical stainings revealed the neoplastic cells positive for CD3 and negative for CD20, CD30 and myeloperxidase (Fig. 4E). In situ hybridization for Epstein-Barr virus (EBV) showed scattered positive cells (Fig. 4F). These histologic, immunohistochemical, and molecular findings confirmed the diagnosis of angioimmunoblastic T-cell lymphoma (AITL).
Therefore, the possibility of early stage lymphoma was suggested.
Colorectal lymphomas are rare. They comprise 5.8% of all cases of gastrointestinal lymphoma and 0.16% of all cases of colon cancer [3].
Primary mesenteric malignant lymphoma is very rare and only 32 cases have been previously reported in Japan [4].
In addition, synchronous adenocarcinoma and lymphoma of the large bowel in the same patient are extremely rare and restricted to a few case reports. In a study of 45 primary lymphomas of the colorectum by Shepherd et al. [5], these accounted for only 0.2% of primary malignant tumors of the colorectum excised at Saint Mark's hospital during a 50-year period. Barron and Localio [2] showed the possibility of the joint occurrence of colon cancer and lymphoma occurring by chance is less than 0.0002%. Recently, there are more reports of synchronous lymphoma and colorectal malignancy. Kidd et al. [6] mentioned these kinds of cases; colon cancer with mantle cell lymphoma, coexisting colon cancer and lymphoma in an IgA-deficient boy and primary malignant lymphoma and colon cancer in the gastrointestinal tract, metastatic colon cancer and follicular lymphoma within the same lymph node [6].
Our case is synchronous primary colon cancer in the ascending colon with malignant AITL in the pericolic lymph node, and there is no other report up to now.
Compared to the third case of Barron and Localio [2], our case is similar in that it is a stumbled-upon mixed-cell type lymphoma after colon cancer surgery. But, we are uncertain whether it is the same with our case in that the lymphocytic-lymphoblastic type was proven after bone marrow biopsy.
AITL is a peripheral T-cell lymphoma characterized by systemic disease, a polymorphous infiltrate involving lymph nodes, with a prominent proliferation of high endothelial venules and follicular dendritic cells. It was previously felt to be an atypical reactive process, angioimmunoblastic lymphadenopathy, with an increased risk of progression to lymphoma. Currently, overwhelming evidence suggests that AITL arises de novo as a peripheral T-cell lymphoma. It is one of the more common specific subtypes of peripheral T-cell lymphoma, accounting for approximately 15 to 20% of cases. The nearly constant association with EBV has suggested a possible role for the virus in the etiology, but the neoplastic T cells are EBV negative. It is clinically characterized by a high fever, generalized lymphadenopathy, and a skin rash. In addition, spleen, liver and bone marrow are frequently involved [7]. Although there is no definite diagnostic criteria for AITL, Mourad et al. [8] reported 5 criteria for histologic diagnosis: partial or diffuse effacement of the nodal architecture, vascular proliferation with prominent arborization of high endothelial venules, extrafollicular meshwork of follicular dendritic cells, atypical population of CD3+ T cells, and large CD20+ B cells. Among them, our case satisfied 4 criteria only, excepting large CD20+ B cells.
When a patient is suspected to have lymphoma, staging evaluation for lymphoma includes physical examination, documentation of B symptoms, laboratory evaluation (complete blood counts, liver function tests, uric acid, calcium), serum protein electrophoresis, serum β2-microglobulin, chest radiograph, CT scan of abdomen, pelvis, and usually chest, bone marrow biopsy, lumbar puncture in lymphoblastic, Burkitt's and diffuse large B cell lymphoma with positive marrow biopsy, gallium scan (single photon emission computed tomography, SPECT) or positron emission tomography (PET) scan in large B cell lymphoma [9]. Among them, our case didn't carry out chest CT, serum β2-microglobulin, bone marrow biopsy, SPECT and PET scan due to patient's refusal. There was no evidence suggesting lymphoma in other studies.
There have been studies that proposed possible factors and mechanisms that may play a role in the occurrence of synchronous colonic carcinoma and lymphoma [4]. The implicating factors include environmental agents, immune abnormalities, and genetic constitution of the patients [10]. Barron and Localio [2] suggested that the lymphomatous process may be the initial event that compromises the patient's immune defenses against the development of colon cancer.
In our case, she had not any specific familial, medical and social history. There was no alternative factor suggested above in this patient. She was very old age and had advanced colon cancer. She had chronic illness including anemia. So, she seemed to have weak immune function. This may implicate the synchronous occurrence of these two tumors, likely due to chance rather than any specific association.
In conclusion, coexisting primary malignant lymphoma and colon adenocarcinoma in one patient is a very rare event. We have no attractive hypothesis to explain the synchronous occurrence of malignant lymphoma and adenocarcinoma in this patient. We consider that old age and decreased immunity may be the risk factors for this case.
Figures and Tables
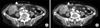 | Fig. 1(A, B) Contrast enhanced computed tomography scan shows irregular heterogeneous wall thickening in cecum and terminal ileum (arrowheads). There were several enhancing pericolic lymph nodes, suggesting metastasis (arrows). |
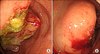 | Fig. 2(A, B) Colonoscopic finding revealed hyperemic lobulated ulcerofungating mass lesion in cecum, which was nearly obstructing cecal lumen. The mass was extened into ileum, but the origin was uncertain. |
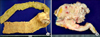 | Fig. 3(A) Grossly, specimen was revealed as ulceroinfiltrative tumorous lesion in cecum, measuring about 7 × 5 cm. (B) Cut surface showed tumor infiltration to the pericolic and mesenteric adipose tissue. |
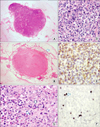 | Fig. 4(A) Non-neoplastic pericolic lymph node with preserved architecture (H&E, ×10). (B) Lymph node with lymphoma involvement. There is subtotal architectural effacement with perinodal infiltration (arrow) (H&E, ×10). (C) Presence of reactive cells including follicular dendritic cells (arrow) and eosinophils (arrowhead) are noted (H&E, ×400). (D) The lymph node was replaced by atypical polymorphous lymphoid cells (H&E, ×400). (E) Atypical lymphoid cells were CD3 (T lymphocytic marker) positive (immunohistocemical staining, ×400). (F) In situ hybridization for Epstein-Barr virus was nuclear positive in reactive B-immunoblasts (arrow) (×400). |
References
1. Koh SJ, Kim JS. The reasons for the increased incidence of colorectal cancer in Korea. Korean J Med. 2010. 79:97–103.
2. Barron BA, Localio SA. A statistical note on the association of colorectal cancer and lymphoma. Am J Epidemiol. 1976. 104:517–522.
3. Romaguera J, Hagemeister FB. Lymphoma of the colon. Curr Opin Gastroenterol. 2005. 21:80–84.
4. Nishimura Y, Takenaka H, Yoshidome K, Iwase K, Oshima S, Tanaka T. Primary mesenteric tumor of adult T-cell leukemia/lymphoma: report of a case. Surg Today. 1994. 24:263–267.
5. Shepherd NA, Hall PA, Coates PJ, Levison DA. Primary malignant lymphoma of the colon and rectum. A histopathological and immunohistochemical analysis of 45 cases with clinicopathological correlations. Histopathology. 1988. 12:235–252.
6. Kidd LR, Evans MD, Williams NW, Beynon J. Synchronous diagnosis of colorectal malignancy and lymphoma. Colorectal Dis. 2011. 13:1107–1109.
7. Dogan A, Gaulard P, Jaffe ES, Ralfkiaer E, Muller-Hermelink HK. Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. Angioimmunoblastic T-cell lymphoma. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon: IARC;309–311.
8. Mourad N, Mounier N, Briere J, Raffoux E, Delmer A, Feller A, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2008. 111:4463–4470.
9. Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J. Harrison's principles of internal medicine. 2011. 18th ed. New York: McGraw Hill.
10. Quilon JM, Day S, Lasker JC. Synchronous tumors: Hodgkin disease presenting in mesenteric lymph nodes from a right hemicolectomy for colon carcinoma. South Med J. 2004. 97:1133–1135.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download