Abstract
Pneumocystis carinii pneumonia (PCP) has rarely been reported in solid tumor patients. It is a well-known complication in immunosuppressed states including acquired immune deficiency syndrome and hematologic malignancy. PCP has been reported in solid tumor patients who received long-term steroid treatment due to brain or spinal cord metastases. We found 3 gastric cancer patients with PCP, who received only dexamethasone as an antiemetic during chemotherapy. The duration and cumulative dose of dexamethasone used in each patient was 384 mg/48 days, 588 mg/69 days, and 360 mg/42 days, respectively. These cases highlight that the PCP in gastric cancer patients can successfully be managed through clinical suspicion and prompt treatment. The cumulative dose and duration of dexamethasone used in these cases can be basic data for risk of PCP development in gastric cancer patients during chemotherapy.
Pneumocystis carinii pneumonia (PCP) usually occurs in patients with acquired immune deficiency syndrome [1]. It can also develop in patients with hematologic malignancy, who received stem cell transplantation [2]. It has been rarely reported in solid cancer patients who underwent standard dose chemotherapy [3]. We experienced three patients with PCP that developed during chemotherapy for gastric cancer. These cases can show us that clinical suspicion is important for early detection and proper management of PCP even during standard dose chemotherapy for gastric cancer.
The patient (female/46) was diagnosed simultaneously with gastric cancer and Krukenberg tumor in February 2010. She received chemotherapy with a regimen of oxaliplatin/fluorouracil/leucovorin combination (mFOLFOX6). Treatment was repeated in two-week intervals. Premedication for antiemesis composed of dexamethasone 8 mg on days 1, 2, and 3 and serotonin 5-HT3 antagonist (ramosetron) on days 1, 2, and 3. She completed 12 cycles of chemotherapy with a regimen of mFOLFOX6 in August, 2010. Two months after the completion of chemotherapy, her cancer progressed in October, 2010. She began to receive a second line of chemotherapy with a regimen of irinotecan/fluorouracil/leucovorin (mFOLFIRI) biweekly. The prophylactic antiemetic regimen was the same as the previous chemotherapy. After the second line of chemotherapy, we decided to quit chemotherapy and focus on supportive care because of a newly developed multiple-bone metastasis. She began to complain of dyspnea and cough without fever 12 days after 4th cycle of chemotherapy. Respiratory sound was normal without crackle or wheezing. Arterial blood gas analysis showed hypoxia and hypocapnia. There was increased reticular opacification on both lower lobes of the lungs on patient's chest X-ray. The chest computed tomography (CT) had a patchy ground-glass appearance. We promptly started treatment of PCP with intravenous sulfamethoxazole/trimethoprim. Prednisolone was added to decrease inflammation. The dyspnea and cough resolved quickly from the beginning of treatment. The treatment continued for 14 days. The multiple-patch ground-glass appearance was nearly completely resolved on chest CT (Fig. 1). Cytomegalovirus polymerase chain reaction (CMV PCR) was negative. PCP PCR was positive but PCP stain (Gomori methenamine silver stain) was negative with induced sputum. Bronchoalveloar lavage (BAL) was not performed due to the patient's poor performance status. Human immunodeficiency virus (HIV) antibody testing was negative. Tests for bacteria and fungus were all negative.
Diagnosis: gastric cancer with Krukenberg tumor, multiple bone metastases.
1st line palliative chemotherapy (mFOLFOX6 12 cycles): 2010.4.1 to 2010.9.9.
2nd line palliative chemotherapy (mFOLFIRI 4 cycles): 2010.11.18 to 2010.12.30.
PCP treatment (trimethoprim/sulfamethoxazole): 2010. 1.13 to 2011.1.24.
Cumulative dose of dexamethasone: 384 mg.
Total days using dexamethasone as antiemetic: 48 days.
Interval from PCP to the last dose of dexamethasone: 12 days.
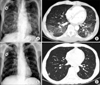
The patient (male/58) underwent total gastrectomy with D2 lymph node dissection for gastric cancer in March, 2007. Six cycles of adjuvant chemotherapy including Epirubicin, 5-fluorouracil and cisplatin (ECF regimen) were performed. Antiemetic regimen composed of intravenous dexamethasone 10 mg at days 1, 2, and 3 and serotonin 5-HT3 antagonist at days 1, 2, and 3. During follow up, his cancer recurred. He was treated with palliative chemotherapy with a regimen of mFOLFOX6. After the 12 cycles of chemotherapy were completed, the disease progressed. Second line palliative chemotherapy with a regimen of mFOLFIRI was administered. An antiemetic regimen for both regimens composed of dexamethasone 8 mg at days 1, 2, and 3 and serotonin 5-HT3 receptor antagonist was given. Dyspnea, hypotension and tachycardia without fever occurred 12 days after the 5th cycle of chemotherapy. Chest CT scan showed patchy ground-glass opacifications throughout both lower lobes. PCP was suspected by clinical symptoms and chest CT scan. Empirical treatment with intravenous trimethoprim/sulfamethoxazole and solumedrol was administered to the patient promptly. After 14 days of treatment, his symptoms resolved and the patchy ground-glass opacifications nearly completely disappeared on chest CT scan (Fig. 2). CMV PCR was negative. PCP PCR was positive. HIV antibody test was negative. PCP stain was negative with induced sputum. BAL was not performed due to his dramatic clinical response to anti-PCP therapy.
Diagnosis: stomach cancer with peritoneal seeding and lymph node metastases.
Total gastrectomy with lymph node dissection: 2007. 3.28.
Adjuvant chemotherapy (ECF 6 cycles): 2007.4.24 to 2007.9.11.
Relapse: 2010.3.
1st line palliative chemotherapy (mFOLFOX6 12 cycles): 2010.4.5 to 2010.9.10.
2nd line palliative chemotherapy (mFOLFIRI 5 cycles): 2010.10.8 to 2010.12.3.
Anti PCP therapy: 2010.12.17 to 2011.1.3.
Cumulative dose of dexamethasone: 588 mg.
Total days using dexamethasone as antiemetic: 69 days.
Interval from PCP to the last dose of dexamethasone: 13 days.
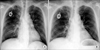
The patient (male/57) was diagnosed as gastric cancer with liver metastasis in March 2006. He received chemotherapy with TS-1 (oral 5-fluorouracil [5-FU]) plus cisplatin combination regimen. His disease progressed after 4 cycles of TS-1 plus cisplatin chemotherapy. Second line palliative chemotherapy with 5-FU/leucovorin/oxaliplatin (mFOLFOX6) was administered. Progression of his disease was found after 7 cycles of mFOLFOX6 chemotherapy. Docetaxel single was administered to him from February 2007 as a palliative treatment. The antiemetic regimen for TS-1 plus cisplatin was 10 mg intravenous at days 1, 2, and 3, plus serotonin 5-HT3 receptor antagonist from days 1 to 5. Antiemetic regimen for mFOLFOX was the same as for TS-1 plus cisplatin. The pemedication for docetaxel was 8 mg dexamethasone the night before chemotherapy, 10 mg at day 1 and 8 mg bid at days 2 and 3 to prevent hypersensitivity reaction of docetaxel. He was admitted with the chief complaint of dry cough and fever 13 days after the 3rd cycle of docetaxel chemotherapy. Chest CT revealed bilateral multifocal ground-glass opacity with some small cystic change. Empirical treatment with trimethoprim/sulfamethoxazole was started immediately. Anti-PCP therapy was changed to pentamidine due to liver toxicity from trimethoprim/sulfamethoxazole. The change in chest X-ray was shown in Fig. 3. BAL was performed. CMV PCR with BAL fluid was negative. Both PCP PCR and PCP stain with BAL fluid was positive. HIV antibody test was negative.
Diagnosis: gastric cancer with liver and peritoneal metastases.
1st line palliative chemotherapy (TS-1 plus cisplatin 4 cycles): 2006.4.8 to 2006.7.25.
2nd line palliative chemotherapy (mFOLFOX6 7 cycles): 2006.7.25 to 2006.11.25.
3rd line palliative chemotherapy (Docetaxel 5 cycles): 2007.2.28 to 2007.5.30.
Anti-PCP therapy: 2010.4.25 to 2010.5.9.
Cumulative dose of dexamethasone: 360 mg.
Total days using dexamethasone as antiemetic: 42 days.
Interval from PCP to the last dose of dexamethasone: 13 days.
The cause of PCP in solid cancer could be both from the use of dexamethasone as antiemetic and weak immunity from the cancer itself. Patients who receive frequent immunosuppressive drugs can develop clinical manifestations of PCP when the corticosteroids are tapered [4]. PCP must be ruled out in these patients, and specific anti-PCP therapy should be used in patients who have their corticosteroids tapered even before the BAL results are known [5]. Therefore, high suspicion and early empirical anti-PCP therapy is important for management of PCP when PCP is clinically suspected.
We reported here 3 cases of PCP in gastric cancer patients who did not receive long-term steroids except for short-term use of dexamethasone as an antiemetic. One case was proven with histological diagnosis. The other two cases were not proven with histological diagnosis. Two patients were diagnosed with probable PCP because the results of PCP PCR were positive and anti-PCP therapy improved clinical symptoms and infiltration of the lungs. All three patients were successfully treated owing to prompt suspicion and timely therapy. The first point that we emphasize is that PCP should be suspected even in gastric cancer patients. The second point is that empirical anti-PCP therapy should not be delayed to manage PCP successfully and to avoid fatal complications even though a confirmatory diagnosis is not made. Patients with especially solid tumor tend to get negative results in histological stain for PCP cyst due to small burden, except for AIDS patients [6-9].
PCP in gastric cancer patients has not been highlighted clinically. During chemotherapy for solid tumors, the National Comprehensive Cancer Network (NCCN) guideline for antiemesis recommends dexamethasone from moderate emetic risk of chemotherapy [10]. Cumulative dose and total days of dexamethasone use could be substantial. In our cases, cumulative dose of dexamethasone was 360 mg, 384 mg, and 584 mg, respectively. Each total days of dexamethasone use was 42, 48, and 69 days, respectively. Attention to PCP even in gastric cancer patients should be warranted even though they just received the normal scheduled dose of dexamethasone as antiemetic.
Almost all solid cancer patients usually receive dexamethasone as an antiemetic. The longer duration of survival and chemotherapy of gastric cancer patients are, the longer the duration and cumulative dose of dexamethasone are. Even though the patients did not receive long-term steroid treatment due to brain or spinal cord metastasis, just routine antiemetic dexamethasone could cause lethal PCP.
Here, we reported PCP patients who successfully recovered with timely suspicion and treatment. The cumulative dose and duration of dexamethasone used in these cases can be used as basic data for risk of PCP development in gastric cancer patients during chemotherapy. We would like to emphasize that if atypical pneumonia is suspected in gastric cancer patients, PCP should be added as a differential diagnosis.
Figures and Tables
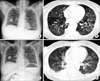 | Fig. 1Chest posterior-anterior view and computed tomography (CT) of patient 1. A and B is the chest X-ray and CT of before anti-pneumocystis carinii pneumonia (PCP) treatment and C and D is that of after anti-PCP treatment respectively. |
ACKNOWLEDGEMENTS
This work was supported by the Konkuk University Medical Center Research Grant 2010.
References
1. Arend SM, Kroon FP, van't Wout JW. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993: an analysis of 78 cases. Arch Intern Med. 1995. 155:2436–2441.
2. Gal AA, Plummer AL, Langston AA, Mansour KA. Granulomatous Pneumocystis carinii pneumonia complicating hematopoietic cell transplantation. Pathol Res Pract. 2002. 198:553–558.
3. Varthalitis I, Aoun M, Daneau D, Meunier F. Pneumocystis carinii pneumonia in patients with cancer: an increasing incidence. Cancer. 1993. 71:481–485.
4. Barbounis V, Aperis G, Gambletsas E, Koumakis G, Demiris M, Vassilomanolakis M, et al. Pneumocystis carinii pneumonia in patients with solid tumors and lymphomas: predisposing factors and outcome. Anticancer Res. 2005. 25(1B):651–655.
5. Walzer P. Mandell GL, Douglas RG, Bennett JE, editors. Pneumocystis carinii. Principles and practice of infectious disease. 1989. New York: Churchill Livingstone;2103–2110.
6. Jacobs JA, Dieleman MM, Cornelissen EI, Groen EA, Wagenaar SS, Drent M. Bronchoalveolar lavage fluid cytology in patients with Pneumocystis carinii pneumonia. Acta Cytol. 2001. 45:317–326.
7. Limper AH, Offord KP, Smith TF, Martin WJ 2nd. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989. 140:1204–1209.
8. Shelhamer JH, Gill VJ, Quinn TC, Crawford SW, Kovacs JA, Masur H, et al. The laboratory evaluation of opportunistic pulmonary infections. Ann Intern Med. 1996. 124:585–599.
9. Thomas CF Jr, Limper AH. Pneumocystis pneumonia: clinical presentation and diagnosis in patients with and without acquired immune deficiency syndrome. Semin Respir Infect. 1998. 13:289–295.
10. Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist. 2007. 12:1143–1150.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download