Abstract
Purpose
Radiofrequency ablation (RFA) is an accepted treatment option for primary and metastatic liver tumors. As percutaneous RFA has some limitations, laparoscopic RFA (LRFA) has been used as a therapeutic alternative for the treatment of hepatic malignancies.
Methods
Between March 2006 and September 2009, thirty patients with hepatic malignancies that were contraindicated for resection or percutaneous RFA underwent LRFA. Indications for this procedure were hepatocellular carcinoma (HCC, 21 patients), metastatic liver tumor (8 patients) and intrahepatic cholangiocarcinoma (1 patient).
Results
Among the 30 patients who underwent LRFA, 5 patients underwent concomitant laparoscopic liver resection. Intraoperative laparoscopic ultrasound detected new malignant lesions in 4 patients (13.3%). A total of 46 lesions were ablated by LRFA. There was no postoperative mortality. The three-year overall survival rate was 83.7% for the HCC group and 64.3% for the metastatic group.
Hepatic resection is the most effective treatment for patients with primary or metastatic hepatic malignancies [1]. Liver resection is usually limited by the poor functional reserve of the liver in cirrhotic patients, multifocal bilateral lesions and tumor proximity to major vascular or biliary structures [2]. Recently, percutaneous radiofrequency ablation (RFA) has gained widespread acceptance as treatment for primary and metastatic liver cancers, especially when liver resection is not well-indicated [3-5]. However, this procedure has limitations when the lesion is located close to a visceral organ (e.g., the gall bladder, stomach, and colon) or is located on the liver surface or the diaphragm [2]. For these patients, laparoscopic RFA (LRFA) can offer a better and effective treatment as it provides for a safer approach to liver lesions difficult or impossible to treat with a percutaneous approach [6-10].
The aim of this study was to evaluate the safety and efficacy of LRFA for primary and metastatic hepatic malignances. Perioperative morbidity, mortality, overall cumulative survival and recurrence rates were retrospectively evaluated.
Between March 2006 and September 2009, 30 consecutive patients with hepatic malignancies underwent LRFA. LRFA was performed in patients not amenable to liver resection or percutaneous RFA. Indications for LRFA were as follows: 1) difficult location of the tumor for performing a percutaneous approach such as close proximity to the diaphragm, gallbladder, stomach, or colon (n = 17), 2) multiple tumors requiring simultaneous laparoscopic liver resection and LRFA (n = 5), 3) multiple lesions requiring repeated punctures (n = 4), 4) superficial lesions which are prone to rupture (n = 3), and 5) simultaneous laparoscopic treatment for both primary colorectal cancer and metastatic liver tumors (n = 1).
Procedures were performed with the patients under general anesthesia in the supine position. A Veress needle was inserted for insufflation of the abdominal cavity. After inserting an 11-mm trocar beneath the umbilicus, a pneumoperitoneum was performed with insufflations of CO2 gas. Abdominal pressure was maintained under 13 mmHg. First, complete inspection of the intraperitoneal organs was performed to rule out any extrahepatic disease. Then another 11-mm trocar was inserted in the epigastric area. A 30 degree or flexible laparoscope was used and ligaments around the liver were dissected using a Harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) to mobilize the liver and to identify the liver lesions. If necessary, adhesiolysis was performed between the liver and other adjacent organs.
All patients underwent LRFA guided by laparoscopic ultrasonography (Aloka Inc., Tokyo, Japan). Liver lesions were identified and the vascularity of the lesions was assessed using the transducer. The puncture site of the radiofrequency electrode was determined using the laparoscopic transducer through an 11-mm trocar placed beneath the xiphoid process. After the lesions were identified and measured, the radiofrequency electrode was placed percutaneously into the lesion and then prongs were deployed. A RF 3000 Radiofrequency Ablation System (Boston Scientific Co., Natick, MA, USA) was used as the energy source. A Cool-tip RF Ablation System (Valleylab, Boulder, CO, USA) was also used with an internally cooled electrode, Cool-tip RF Electrode. Laparoscopic ultrasound (LUS) examination and RFA procedures were performed by radiologists. Ablation was repeated until the whole tumor showed highly echogenic changes.
The primary effectiveness of the treatment was evaluated with a follow-up contrast-enhanced study one month after LRFA. Incomplete ablation was defined as the appearance of irregular peripheral-enhancing foci around the ablation zone in the follow-up images, while complete necrosis of the index tumor was defined by the absence of an enhanced tumor area. Local tumor progression was determined to exist when a subsequent follow-up computed tomography (CT) demonstrated the presence of a growing and enhanced tumor in the ablation zone where there had been complete primary effectiveness. New lesion tumor progression was defined as a growing and enhanced tumor occurring away from the ablation zone.
Of the 30 patients, 21 patients were male and 9 patients were female. The mean age of the patients was 58.8 years (range, 35 to 76 years). Forty-six LRFA procedures were performed to ablate primary or metastatic hepatic tumors in 30 patients. There were 21 patients with hepatocellular carcinoma (HCC), 8 patients with metastatic hepatic malignancies and one patient with cholangiocarcinoma who was suspected of having HCC preoperatively. Of the 8 patients with metastatic hepatic malignancies, the most common primary malignancy was colorectal cancer (n = 5). Other malignancies were breast cancer (n = 1), renal cell carcinoma (n = 1) and Klatskin tumor (n = 1). Of the 8 patients with metastatic hepatic malignancies, metachronous metastasis was dominant (n = 7).
The Child-Pugh class of most patients with HCC was class A (85.7%). The most common cause of HCC was hepatitis B (85.7%). All patients with HCC underwent transarterial chemoembolization or percutaneous RFA preoperatively. Of the 30 patients, 14 patients (46.7%) underwent additional laparoscopic surgery simultaneously with LRFA procedures: hepatic resection in 5 (4 tumorectomies and 1 left lateral sectionectomy), lower anterior resection in one and cholecystectomy in 8 patients (Table 1).
Nineteen patients underwent LRFA for only one lesion. Another six and five patients had LRFA for 2 and 3 lesions, respectively. A median tumor size was 2.0 cm (range, 0.8 to 4.5 cm). New malignant lesions including 3 HCCs and 1 metastatic tumor were identified by LUS, which were not detected by preoperative ultrasound, CT or magnetic resonance imaging (MRI). These newly detected nodules were ablated with LRFA.
The mean operation time was 195.0 minutes and the mean intraoperative blood loss was 240.7 mL. Transfusion was required in three patients (10.0%) during the operation. The mean postoperative hospital stay was 7.4 days. There was no operative mortality. Postoperative complications were observed in 7 of 30 patients (23.3%) including right pleural effusion in 3 patients, and perihepatic fluid collection, mild ascites, hydropneumothorax and hematoma at the puncture site in 1 patient respectively. All patients with complications recovered well with conservative management (Table 2).
The median follow-up period was 18.2 months (range, 3.4 to 41.1 months). The overall success rate for LRFA was 95.7% and the three-year overall survival rates of patients with HCC and metastatic tumors were 83.7% and 64.0%, respectively (Fig. 1). One patient with cholangiocarcinoma died of multiple intraperitoneal metastases 10 months after LRFA.
During follow-up in 21 patients with HCC, 13 patients (61.9%) experienced a recurrence. The overall recurrence rate at 3, 6, and 12 months after LRFA was 14.3%, 19.6%, and 47.4%, respectively (Fig. 2). The median disease-free survival time was 11.6 months (range, 1.0 to 36.3 months). There were 2 cases of incomplete ablation, 2 cases of local tumor progression and 12 cases of new intrahepatic lesions. Two patients had both local tumor progression and development of a new lesion. One patient had both incomplete ablation and a new lesion (Table 3).
In the follow-up of 8 patients with metastatic hepatic malignancies, 5 patients (62.5%) had recurred lesions. The cumulative overall recurrence rate at 3, 6, and 12 months after the LRFA was 12.5%, 37.5%, and 53.1%, respectively (Fig. 2). The median disease-free survival time was 9.4 months (range, 1.6 to 34.5 months). Of these 5 patients, 3 patients had tumor recurrences within the liver. One case had both incomplete ablation and local tumor progression, and two cases had new tumor progression. One patient had extrahepatic tumor recurrence in the lung and the another patient had both intrahepatic tumor recurrence and extrahepatic lymph node metastasis.
RFA of primary and metastatic hepatic malignancies by laparoscopy can be a useful substitute for percutaneous RFA when the lesions are superficial or close to visceral organs [11]. In a study of LRFA for 66 patients with HCC by Berber et al. [12], the overall survival rates at 1, 2 and 3 years were 78%, 48% and 38%, respectively. Curley et al. [13] reported a 1.8% of local tumor progression rate and a 21.9% of new tumor recurrence rate in 48 patients with HCC and 75 patients with metastatic hepatic malignancies during follow-up (mean, 15.0 months). In contrast, the mean overall survival rate for the patients with unresectable metastatic malignancies was only 9 to 14 months [14,15]. In our study, 3-year survival rates for HCC and metastatic hepatic malignancies were 83.7% and 64.3%, respectively while one-year cumulative recurrence rates for HCC and metastatic hepatic malignancies were 47.4% and 53.1%, respectively. There was no operative mortality. Our results showed a better survival and a similar recurrence rate in comparison with the aforementioned other studies.
The LRFA procedure has been reported to be safe for treating hepatic malignancies [16-18]. In a study by Berber and Siperstein [19], a total of 521 RFA procedures were performed for 428 patients (286 men and 142 women); these patients had a mean age of 61 years (range, 25 to 89 years). A total of 1,636 lesions (mean, 3.1 per patients with a range, 1 to 16) were ablated. The 30-day mortality rate was 0.4% (n = 2) and the morbidity rate was 3.8% (n = 20). The post RFA complications were self-limited nausea, ileus, abdominal distention, abdominal pain, fevers and urinary retention in 6 patients and liver abscess in 4 patients. There were also trocar injuries, postoperative bleeding, pneumonia, wound infection and variceal bleeding. In our study, there was no postoperative mortality. Seven patients had postoperative morbidity (pleural effusion, ascites, hydropneumothorax, fluid collection and hematoma), which were treated with conservative management. During the follow-up, other complications including liver abscess did not occur.
Jung et al. [9] introduced LRFA as a useful procedure for the treatment of HCC regardless of its location. According to their reports, serum transaminase levels were transiently elevated (P < 0.01) after RFA but returned to normal within one week, and alpha-fetoprotein level decreased significantly 1 month after RFA (P < 0.05). And the results showed that the LRFA procedure is a good treatment for severe cirrhosis patients with reducing deterioration of liver function. Our study showed that LRFA was good approach method for superficial lesions in the liver or lesions close to visceral organs with the result of 16.7% of local tumor progression rate. LUS is a useful and essential diagnostic tool in LRFA. Santambrogio et al. [20] reported that during the LRFA for HCC, intraoperative LUS identified 26 new malignant lesions (25%) which had been missed by preoperative imaging. We detected new nodules in 4 patients (13.3%, 2 HCCs, and 2 metastatic tumors) using LUS and performed additional ablation for them. In particular, lesions which were small-sized and hard to detect with CT or MRI were able to be detected with LUS. By using LUS, both accurate staging and effective treatment were able to be achieved.
One of the important roles of LRFA is simultaneous use of this procedure with laparoscopic liver resection. When a patient had multiple lesions, the main lesions were resected with laparoscopic liver resection and the remaining small lesions were treated with LRFA. Therefore, application of laparoscopic liver resection has been increasing and the role of LRFA is increased in the treatment of hepatic malignancies [21-24]. In our study, 5 patients underwent laparoscopic liver resection and LRFA simultaneously. With a mean follow-up time of 24.2 ± 14.3 months, they are all alive without specific complications.
Recently, RFA has been reported as a good bridge therapy prior to liver transplantation: the ablation success rate with this procedure is 91.4%. In particular, the laparoscopic approach is associated with a lower adhesion than open surgery and dissection around the liver hilum is not required in this approach [25]. Thus, LRFA would be a good bridge therapy for prevention of tumor progression and downstaging of multiple lesions.
In conclusion, in cases not amenable to surgical resection, LRFA represents a safe and effective treatment for malignant liver tumors especially when a percutaneous approach to the lesions is deemed difficult.
Figures and Tables
 | Fig. 1Overall survival rates after laparoscopic radiofrequency ablation for primary hepatocellular carcinoma (A) and liver metastases (B). 3YRS, 3 year overall survival rates. |
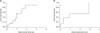 | Fig. 2Cumulative recurrence rates after laparoscopic radiofrequency ablation for primary hepatocellular carcinoma (A) and liver metastases (B). |
Table 3
Recurrence patterns for laparoscopic radiofrequency ablation in patients with hepatocellular carcinoma (HCC) and metastases

In HCC patients, 4 patients had both intrahepatic and extrahepatic recurrences, 2 patients had both local and new lesion formation and 1 patient had both incomplete ablation and new lesion formation. In the metastasis patients, 1 patient had both incomplete ablation and local recurrence and 1 patient had a new lesion and extrahepatic recurrence.
References
1. Clark HP, Carson WF, Kavanagh PV, Ho CP, Shen P, Zagoria RJ. Staging and current treatment of hepatocellular carcinoma. Radiographics. 2005. 25:Suppl 1. S3–S23.
2. Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003. 237:171–179.
3. Wang XH, Cheng F, Zhang F, Li XC, Qian JM, Kong LB, et al. Living donor liver transplantation treatment of Wilson's disease complicated with neuropathy. Zhonghua Yi Xue Za Zhi. 2003. 83:1569–1571.
4. Siperstein A, Garland A, Engle K, Rogers S, Berber E, String A, et al. Laparoscopic radiofrequency ablation of primary and metastatic liver tumors. Technical considerations. Surg Endosc. 2000. 14:400–405.
5. Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001. 221:447–454.
6. Chung MH, Wood TF, Tsioulias GJ, Rose DM, Bilchik AJ. Laparoscopic radiofrequency ablation of unresectable hepatic malignancies: a phase 2 trial. Surg Endosc. 2001. 15:1020–1026.
7. Cuschieri A, Bracken J, Boni L. Initial experience with laparoscopic ultrasound-guided radiofrequency thermal ablation of hepatic tumours. Endoscopy. 1999. 31:318–321.
8. Goletti O, Lencioni R, Armillotta N, Puglisi A, Lippolis PV, Lorenzetti L, et al. Laparoscopic radiofrequency thermal ablation of hepatocarcinoma: preliminary experience. Surg Laparosc Endosc Percutan Tech. 2000. 10:284–290.
9. Jung MK, Lee JH, Kim TS, Kim HS, Cho CM, Tak WY, et al. Laparoscopic and percutaneous ultrasound guided radiofrequency ablation for hepatocellular carcinoma: a preliminary study. Korean J Hepatol. 2002. 8:209–217.
10. Santambrogio R, Bianchi P, Pasta A, Palmisano A, Montorsi M. Ultrasound-guided interventional procedures of the liver during laparoscopy: technical considerations. Surg Endosc. 2002. 16:349–354.
11. Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005. 242:158–171.
12. Berber E, Rogers S, Siperstein A. Predictors of survival after laparoscopic radiofrequency thermal ablation of hepatocellular cancer: a prospective study. Surg Endosc. 2005. 19:710–714.
13. Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999. 230:1–8.
14. Rosales J, Leong LA. Chemotherapy for metastatic colorectal cancer. J Natl Compr Canc Netw. 2005. 3:525–529.
15. McMurrick PJ, Nelson H. Liver-directed therapies for gastrointestinal malignancies. Curr Opin Oncol. 1997. 9:367–372.
16. Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1000 cases. Cancer. 2005. 103:1201–1209.
17. Jiang HC, Liu LX, Piao DX, Xu J, Zheng M, Zhu AL, et al. Clinical short-term results of radiofrequency ablation in liver cancers. World J Gastroenterol. 2002. 8:624–630.
18. Kim KH, Yoon YS, Yu CS, Kim TW, Kim HJ, Kim PN, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. 2011. 81:25–34.
19. Berber E, Siperstein AE. Perioperative outcome after laparoscopic radiofrequency ablation of liver tumors: an analysis of 521 cases. Surg Endosc. 2007. 21:613–618.
20. Santambrogio R, Podda M, Zuin M, Bertolini E, Bruno S, Cornalba GP, et al. Safety and efficacy of laparoscopic radiofrequency ablation of hepatocellular carcinoma in patients with liver cirrhosis. Surg Endosc. 2003. 17:1826–1832.
21. Bleicher RJ, Allegra DP, Nora DT, Wood TF, Foshag LJ, Bilchik AJ. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003. 10:52–58.
22. Vlastos G, Smith DL, Singletary SE, Mirza NQ, Tuttle TM, Popat RJ, et al. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004. 11:869–874.
23. Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004. 239:818–825.
24. Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003. 10:1059–1069.
25. Martin AP, Goldstein RM, Dempster J, Netto GJ, Katabi N, Derrick HC, et al. Radiofrequency thermal ablation of hepatocellular carcinoma before liver transplantation: a clinical and histological examination. Clin Transplant. 2006. 20:695–705.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download