Abstract
Purpose
This study investigated the impact of subclinical borderline changes on the development of chronic allograft injury in patients using a modern immunosuppression protocol.
Methods
Seventy patients with stable renal allograft function and who underwent protocol biopsies at implantation, 10 days and 1 year after transplantation were included and classified based on biopsy findings at day 10. The no rejection (NR) group included 33 patients with no acute rejection. The treatment (Tx) group included 21 patients with borderline changes following steroid pulse therapy, and the nontreatment (NTx) group included 16 patients with borderline changes nontreated.
Results
The Banff Chronicity Score (BChS) and modified BChS (MBChS) were not different among the three groups at implantation (P = 0.48) or on day 10 (P = 0.96). Surprisingly, the NTx group had more prominent chronic scores at the 1-year biopsy, including BChS (3.07 ± 1.33, P = 0.005) and MBChS (3.14 ± 1.41, P = 0.008) than those in the Tx and NR group, and deterioration of BChS was more noticeable in the NTx group (P = 0.037), although renal function was stable (P = 0.66). No difference in chronic injury scores was observed between the Tx and NR groups at the 1-year biopsy.
Chronic allograft injury accompanying interstitial fibrosis and tubular atrophy is the most important cause of graft loss after kidney transplantation [1]. Although the incidence of acute rejection decreased significantly with the introduction of potent immunosuppressants, it remains a leading cause of chronic allograft injury among immunologic and nonimmunologic causes. Subclinical rejection detected by early protocol biopsy has also been correlated with subsequent chronic allograft nephropathy and allograft dysfunction [2,3]. Therefore, the Banff 97 working classification of renal allograft pathology was introduced to standardize the histological definition of acute allograft rejection and to guide treatment of renal transplant recipients [4,5]. The Banff scheme defines the minimal threshold for acute T-cell mediated rejection as infiltration of 25% or more of the renal cortex with five or more mononuclear cells in a focus of tubulitis or intimal arteritis (histological indices i2t2 or v1) and refers to borderline changes as those with insufficient for a diagnosis of acute T-cell mediated rejection, including mild to moderate (<50%) cortical infiltration and one to four mononuclear cells per tubule in cross section (i1t1 or i2t1) [6]. Although borderline changes are detected in as many as 38% of protocol biopsies at 1 to 2 weeks posttransplantation [7], the pathogenic role of such limited cortical mononuclear infiltration is not well established. In addition, no consensus for the treating patients with borderline changes has been reached. Borderline changes with graft dysfunction are occasionally routinely treated with steroid pulse and, whereas subclinical borderline changes are simply 'ignored'.
Previous studies addressing the influence of subclinical borderline changes on renal allograft outcome focused on the progression of the borderline changes to acute rejection, and data of long-term impact on renal histology are scarce [8-10]. Furthermore, relevant studies with patients who underwent modern immunosuppressive regimens including tacrolimus and those with early borderline changes at 10 days after transplantation are lacking.
Therefore, the aim of this study was to investigate the clinical significance of the borderline changes identified by early protocol biopsies in terms of chronic allograft injury in the era of tacrolimus-based immunosuppression and to evaluate the effect of steroid pulse therapy in stable renal transplant recipients with subclinical borderline changes.
A total of 248 adult renal transplants were performed at Seoul National University Hospital between January 2008 and August 2010. Unit policy during this period was to perform protocol biopsies at time zero, 10 days and 1 year after transplantation. Patients who underwent all three protocol biopsies with stable graft function were included in this study (n = 70). Any patient who underwent preoperative desensitization (n = 13), who had acute cellular or antibody-mediated rejection on the 10-day protocol biopsy (n = 21), and who showed clinical rejection between protocol biopsies (n = 19) were excluded. Those who had a clinically-indicated biopsy at any time within 1 year after transplantation (n = 51), who did not undergo one or more biopsies of the three protocol biopsies (n = 59), and who had multiple organ transplantation (n = 15) were also excluded. The remaining 70 kidney transplant recipients and 210 biopsies were finally included in this study.
Patients were classified according to the 10-day protocol biopsy result and whether borderline change was treated or not to evaluate the impacts of subclinical borderline changes on allograft injury. Patients who had no clinical rejection, subclinical rejection, or borderline changes until 1 year after transplantation after three protocol biopsies were classified as the no rejection (NR) group (n = 33) as a control. Patients with borderline changes on 10-day protocol biopsies were classified as the treatment (Tx) group (n = 21) or nontreatment (NTx) group (n = 16) based on whether or not they underwent steroid pulse therapy.
This study was conducted in accordance with the Declaration of Helsinki and its amendments. This study protocol was approved by the Institutional Review Board of the Seoul National University Hospital (IRB No. H-1110-081-382).
All recipients received induction therapy with basiliximab and triple maintenance immunosuppression with tacrolimus, mycophenolate mofetil and corticosteroid [11]. The targeted trough level of tacrolimus was 8 to 10 ng/mL for the first 3 months and 5 to 8 ng/mL until 1 year after transplantation. All patients were treated according to this protocol. Mycophenolate mofetil was given at a fixed dose (1,000 to 1,500 mg per day). Prednisolone was administered initially at 10 mg/kg/day with rapid tapering to 5 mg/day within 2 weeks after transplantation. The decision to treat patients with subclinical borderline changes to treat was made based on the attending physician's (JH and SJK) preference. This decision differed by physician, and their preferences did not changed during the study period. Pulsed intravenous steroid therapy (methylprednisolone 0.5 g daily for 3 days) was performed in 21 patients with borderline changes on the 10-day protocol biopsies.
All study patients had stable graft function throughout the study period and at the time of protocol biopsy. Stable function was defined as serum creatinine ≤1.5 mg/dL and ≤15% increase in serum creatinine in the 2 weeks before biopsy to exclude clinical rejection at the time of biopsy. Zero time biopsies were performed using an 18-gauge biopsy gun by a transplant surgeon in the operating room. Protocol biopsies at 10 days and 1 year after transplantation were performed using an 18-gauge needle under ultrasound guidance. Three cores of tissue were obtained at each biopsy. Neither biopsy-related complications nor graft loss occurred.
All biopsies were reviewed by a single pathologist, blinded to the clinical information and the results were classified according to the Banff '97 criteria and its updates [5,12,13]. The chronic changes were graded in a semiquantitative manner to provide a Banff Chronicity Score (BChS), a modified Banff Chronicity Score (MBChS) and Transplant Vascular Damage Score (TVDS) [14]. The BChS was calculated as the sum of scores for the individual histological markers for chronicity: interstitial fibrosis (ci), tubular atrophy (ct), vascular fibrous intimal thickening (cv), and chronic glomurulopathy (cg). The components of MBChS included glomerulosclerosis, ct, ci, and cv. The TVDS was calculated using the components of arterial hyalinosis (ah) and cv.
All data were collected prospectively in the Seoul National University Hospital Transplant Database and retrieved for this study. Statistical analyses (Kruskal-Wallis H, χ2 test, and ordinal logistic regression) were conducted using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Results are presented as mean ± standard deviation or as frequencies (percentages). Ordinal logistic regression analysis was used to assess the effects of risk factors on the development of chronic allograft injury. The confounders for the logistic regression analysis included: age at transplantation, donor age, gender, body mass index, numbers of human leukocyte antigen mismatches, donor type (living or deceased), panel reactive antibody (PRA) status at transplantation, numbers of transplantation procedures (first or retransplant), diabetes mellitus, hypertension, duration of pretransplant dialysis, cytomegalovirus status, and borderline changes on the 10-day biopsy (Tx or NTx). Variables that were significant at the P < 0.10 level in a univariate analysis were included in the final multivariate model by forward stepwise logistic regression.
Clinical data for the NR (n = 33), Tx (n = 21), and NTx (n = 16) groups are shown in Table 1. No significant differences in mean age, male ratio, duration of pretransplant dialysis, number of human leukocyte antigen mismatches, cold ischemic time, donor type (living vs. deceased), PRA level before transplantation, or retransplantation were found among the three study groups. The NR group (39.4 ± 13.3 years) showed a tendency for slightly lower donor age compared to that of the Tx (43.8 ± 15.4 years) and NTx (47.9 ± 9.5 years) groups (P = 0.12). Cold ischemic time for deceased donor transplantation was generally short and less than 5 hours in all groups. Steroid pulse therapy did not induce any significant adverse effects in the Tx group.
All three study groups showed comparable histological features at implantation. No differences in any of the chronic score components such as ci, ct, cv, cg, ah, or mm were observed (all P > 0.05). The BChS, calculated as the sum of scores for ci, ct, cg, and cv were not different among the three groups (0.5 ± 0.89 vs. 0.2 ± 0.7 vs. 0.4 ± 0.5, respectively for the NR, Tx, and NTx groups, P = 0.38). The MBChS (sum of scores for ci, ct, cv and gs) and TVDS (sum of cv and ah) were also not different among the three groups at implantation (P = 0.75 and P = 0.31, respectively).
The study groups were defined by the acute injury scores on the 10-day protocol biopsies. The NR group had no inflammatory lesions and, in some cases, only minimal interstitial inflammation (i1t0 or i2t0). The Tx and NTx groups had borderline changes including mild tubulitis (t1) and interstitial inflammation (i1 or i2). The severity of interstitial inflammation was not different between the Tx and NTx groups (P = 0.62). The early protocol biopsy performed at 10 days after transplantation also revealed no difference in chronic scores among the three groups. Other findings detected on 10-day protocol biopsies were acute tubular necrosis in five patients (one in the NTx group, three in the Tx group and one in the NR group) and calcineurin inhibitor toxicity in two patients (one in the NTx group and one in the NR group).
All study patients had stable graft function until 1 year after transplantation, regardless of the early protocol biopsy results or treatment for borderline changes. No significant impact on renal graft function was observed throughout the study period whether there were borderline changes or not on 10-day protocol biopsies or whether those borderline changes were treated with steroid pulse therapy or not (Fig. 2). Estimated glomerular filtration rates (eGFRs) at 10-day protocol biopsy were 63.6 ± 5.3 mL/min, 58.4 ± 5.3 mL/min, and 59.3 ± 6.6 mL/min for the NR, Tx and NTx groups, respectively (P = 0.76). Patients had stable graft function until the 1-year protocol biopsies, and the respective eGFRs were 64.6 ± 2.7 mL/min, 62.2 ± 3.0 mL/min and 58.2 ± 4.9 mL/min for the NR, Tx and NTx groups at the 1-year protocol biopsy (P = 0.44).
Chronic injury scores were comparable between the time zero and 10-day protocol biopsies in all groups, and all three study groups showed increasing chronic injury scores including BChS and MBChS between the 10-day and 1-year protocol biopsies (Fig. 3). BChS in the NTx group increased from 0.75 ± 0.93 at 10-day to 3.38 ± 1.75 at 1-year (P < 0.001) and MBChS increased from 1.50 ± 1.41 to 3.81 ± 1.80 during the same period (P < 0.001). The Tx group also showed increased BChS (0.48 ± 0.80 to 1.62 ± 1.40, P = 0.002) and MBChS (0.86 ± 0.96 to 2.24 ± 1.67, P = 0.002) during the same period. Interestingly, the NR group also suffered from increased BChS (1.09 ± 1.21 to 1.88 ± 1.27, P = 0.012) and MBChS (1.49 ± 1.39 to 2.15 ± 1.46, P = 0.062). TVDS was not significantly different among the three protocol biopsies in all study groups.
Although all study groups showed increasing chronic injury scores, the NTx group suffered from more prominent chronic injury than that of the other groups (Figs. 4, 5). BChS was significantly higher in the NTx group (3.38 ± 1.75) compared to that in the Tx (1.62 ± 1.40) and NR (1.88 ± 1.27) groups at 1 year after transplantation (P = 0.001). MBChS (3.81 ± 1.80 vs. 2.24 ± 1.67 and 2.15 ± 1.46, P = 0.003) and TVDS (1.31 ± 1.49 vs. 0.43 ± 0.60 and 0.45 ± 0.56, P = 0.004) were also significantly higher in the NTx group compared to those in the Tx and NR groups at the 1-year protocol biopsies (Fig. 4). The chronic injury scores were comparable between the Tx and NR groups in the 1-year protocol biopsies (all P > 0.05).
The changes in BChS, MBChS, and TVDS (dBChS, dMBChS, and dTVDS) between the 10-day and 1-year protocol biopsies were calculated by subtracting the chronic injury scores of the 1-year protocol biopsies from those of the 10-day protocol biopsies (Fig. 5). The NTx group had significantly deteriorated chronic scores between the 10-day and 1-year protocol biopsies and showed worse dBChS (-2.63 ± 2.06) compared with that of the Tx (-1.14 ± 1.32, P = 0.007) and NR group (-0.79 ± 1.50, P < 0.001). dMBChS showed significant deterioration in the NTx group (-2.31 ± 2.33) compared to that in the NR group (-0.67 ± 1.45, P = 0.003). dTVDS was also worse in the NTx group (-0.50 ± 0.97) than that in the NR group (0.24 ± 0.90, P = 0.004). The Tx group and NR groups showed comparable dBChS (P = 0.427), dMBChS (P = 0.145), and dTVDS (P = 0.209).
In the multivariate regression analysis, 'No treatment for borderline change at early protocol biopsy' (odds ratio [OR], 3.796, P = 0.045 for BChS; OR, 3.622, P = 0.05 for MBChS) and donor age (OR, 1.059, P = 0.007 for BChS; OR, 1.057, P = 0.008 for MBChS) were significant risk factors for increasing chronic injury scores at the 1-year protocol biopsies.
The incidence rate of subclinical borderline changes detected by early protocol biopsies was 14.9% in this population which was comparable with previous reports showing incidence rates of 12 to 21.3% [2,16,17]. The decision of whether to treat subclinical borderline changes detected by protocol biopsies should depend on the probability of progression to acute rejection and deterioration of graft function, longevity and prevention of chronic injury, for treating subclinical borderline changes. Despite growing evidence showing chronic renal allograft injuries with subclinical inflammation including borderline changes [18,19], current recommendations are not clearly defined for borderline changes detected by protocol biopsies [20].
We analyzed the clinical course of subclinical borderline changes and evaluated the value of steroid pulse therapy with serial protocol biopsies. The results showed that the borderline changes detected by early (10-day) protocol biopsies negatively influenced the renal allograft histology from the perspective of chronic allograft injury but injuries cannot 'simply' be evaluated by renal allograft function expressed as the GFR. Among the three study groups, which maintained stable renal allograft function, the NTx group showed significantly worse chronic injury scores on the BChS (P = 0.001), MBChS (P = 0.003) and TVDS (P = 0.004) compared to those in the Tx and NR groups at the 1-year protocol biopsies, although all groups had significantly increased chronic histological scores (Fig. 4). The dBChS was calculated by subtracting BChS at the 1-year protocol biopsy from that of the 10-day protocol biopsy and was more aggravated in the NTx group compared with that in the Tx (P = 0.007) and NR groups (P < 0.001) (Fig. 5). Furthermore, 'no treatment for borderline changes at the early protocol biopsy' was identified as a risk factor for aggravating chronic injuries in the multivariate analysis. Therefore, we consequently suggest that subclinical borderline changes should be treated with steroid pulse therapy to prevent chronic allograft injury. This confirmed results by Miyagi et al. [2] who investigated the beneficial effects of high-dose methylprednisolone treatment against borderline changes and nonspecific inflammatory changes detected by early protocol biopsies, in terms of preventing chronic allograft nephropathy. This study also provided a histological interpretation, through multiple time-point protocol biopsies, of recently published study by Thierry et al. [19] showing the presence of subclinical inflammation negatively impacting long-term renal function.
Our results help to unravel previously contradictory results in the literature. Seron et al. [16] reported no difference in mean serum creatinine levels between patients at 3-month biopsies who had normal histology and patients with borderline changes after a follow-up period of 1 and 2 years. The Transplant Group at the University of Chicago also showed that serum creatinine increases >110% of baseline and borderline changes progressed to acute rejection in only 28% of untreated borderline changes; thus, mandatory treatment was not recommended [9]. Roberts et al. [17] reported that subclinical borderline changes detected by protocol biopsies at day 28 were not an adverse prognostic factor for long-term outcome in terms of renal allograft function. However, our results suggest that serum creatinine and clinical allograft function per se may not reflect worsening allograft histology well from the perspective of chronic injury. Furthermore, the majority (76.9%) of patients with borderline changes in reference 16 was in the resolving period of treated acute rejection and the influence of borderline changes on graft outcome could not be precisely examined. Furthermore, patients in previous studies used cyclosporine as the main immunosuppressant so their results may not reflect current immunosuppressive practices.
Our study clearly showed that steroid pulse therapy for subclinical borderline changes was associated with improved allograft histology. This agrees closely with several randomized studies showing better histological and functional outcomes in renal transplant patients with early protocol biopsy and the treatment of subclinical rejection [21]. Some authors may argue that routinely treating borderline changes detected by protocol biopsy may increase opportunistic infections and other potential side effects. Needless to say, modification of immunosuppression to higher intensity with fewest adverse effects is ideal. Tacrolimus can be added to patients who are being maintained with other immunosuppressants. Some investigators have agreed with the practice of withholding antirejection treatment while remaining vigilant for signs of allograft function deterioration [10]. However, all patients in this study were administered the most up-to-date immunosuppressive medications. Although the benefit of routine treatment should be balanced with following risks, in addition, the potential hazards of steroid pulse therapy are relatively acceptable [22].
This study had several limitations. This study is nonrandomized in nature. The study patients were not consecutive and there may be a selection bias. The small sample size was also problematic. However, this study had several strengths. The patient characteristics were well matched, and this homogeneity of the population could have offset selection bias to some extent. Maintenance immunosuppression consisted of a triple regimen including tacrolimus, mycophenolate mofetil, and corticosteroid in all patients, which is the most commonly used treatment regimen in current practice and the tacrolimus trough level was maintained throughout the study period in the 5 to 8 ng/mL which is the currently recommended level (Fig. 1).
In conclusion, our results suggest that subclinical borderline changes detected by early protocol biopsies were associated with chronic renal allograft injury and that the influence of these changes could not be assessed by clinical renal function per se. Treatment of subclinical borderline changes with steroid pulse therapy should be considered to improve chronic allograft histology. Data from prospective and randomized studies using modern immunosuppressants is required to confirm the results of this study and provide clinicians with the confidence to treat subclinical borderline changes.
Figures and Tables
 | Fig. 1Changes in mean tacrolimus (TAC) trough levels. NR, no rejection; Tx, treatment; NTx, nontreatment. |
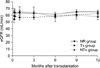 | Fig. 2Changes in renal allograft function. Graft function was not influenced and maintained stable until 1-year, regardless of borderline changes at the 10-day protocol biopsy and whether those borderline changes were treated with steroid pulse therapy or not. NR, no rejection; Tx, treatment; NTx, nontreatment; eGFR, estimated glomerular filtration rate. |
 | Fig. 3Changes in chronic injury scores between protocol biopsies. BChS, Banff Chronicity Score; MBChS, modified Banff Chronicity Score; TVDS, Transplant Vascular Damage Score; 0-D, at implantation; 10-D, at 10-day protocol biopsy; 1-Y, at 1 year protocol biopsy. *P < 0.01. †P > 0.05. ‡P < 0.05. |
 | Fig. 4Comparison of chronic injury scores at the 1-year protocol biopsies. The nontreatment (NTx) group showed high chronic injury scores on all assessments and the treatment (Tx) group showed comparable scores with those of the no rejection (NR) group. BChS, Banff Chronicity Score; MBChS, modified Banff Chronicity Score; TVDS, Transplant Vascular Damage Score. *P < 0.01. †P > 0.05. ‡P < 0.05. |
 | Fig. 5Changes in chronic injury scores (dBChS, dMBChS and dTVDS) calculated by subtracting the 1-year protocol biopsy scores from the 10-day protocol biopsy scores. BChS, Banff Chronicity Score; MBChS, modified Banff Chronicity Score; TVDS, Transplant Vascular Damage Score; NTx, nontreatment; Tx, treatment; NR, no rejection. *P < 0.01. †P > 0.05. ‡P < 0.05. |
References
1. Chapman JR, O'Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005. 16:3015–3026.
2. Miyagi M, Ishikawa Y, Mizuiri S, Aikawa A, Ohara T, Hasegawa A. Significance of subclinical rejection in early renal allograft biopsies for chronic allograft dysfunction. Clin Transplant. 2005. 19:456–465.
3. Legendre C, Thervet E, Skhiri H, Mamzer-Bruneel MF, Cantarovich F, Noel LH, et al. Histologic features of chronic allograft nephropathy revealed by protocol biopsies in kidney transplant recipients. Transplantation. 1998. 65:1506–1509.
4. Furness PN, Kirkpatrick U, Taub N, Davies DR, Solez K. A UK-wide trial of the Banff classification of renal transplant pathology in routine diagnostic practice. Nephrol Dial Transplant. 1997. 12:995–1000.
5. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999. 55:713–723.
6. Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993. 44:411–422.
7. Nankivell BJ, Chapman JR. The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant. 2006. 6:2006–2012.
8. Saad R, Gritsch HA, Shapiro R, Jordan M, Vivas C, Scantlebury V, et al. Clinical significance of renal allograft biopsies with "borderline changes," as defined in the Banff Schema. Transplantation. 1997. 64:992–995.
9. Meehan SM, Siegel CT, Aronson AJ, Bartosh SM, Thistlethwaite JR, Woodle ES, et al. The relationship of untreated borderline infiltrates by the Banff criteria to acute rejection in renal allograft biopsies. J Am Soc Nephrol. 1999. 10:1806–1814.
10. Schweitzer EJ, Drachenberg CB, Anderson L, Papadimetriou JC, Kuo PC, Johnson LB, et al. Significance of the Banff borderline biopsy. Am J Kidney Dis. 1996. 28:585–588.
11. Min SI, Yun IJ, Kang JM, Park YJ, Min SK, Ahn C, et al. Moderate-to-severe early-onset hyperuricaemia: a prognostic marker of long-term kidney transplant outcome. Nephrol Dial Transplant. 2009. 24:2584–2590.
12. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008. 8:753–760.
13. Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant. 2004. 4:1562–1566.
14. Kambham N, Nagarajan S, Shah S, Li L, Salvatierra O, Sarwal MM. A novel, semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. Clin J Am Soc Nephrol. 2007. 2:135–142.
15. Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007. 357:2562–2575.
16. Seron D, Moreso F, Bover J, Condom E, Gil-Vernet S, Canas C, et al. Early protocol renal allograft biopsies and graft outcome. Kidney Int. 1997. 51:310–316.
17. Roberts IS, Reddy S, Russell C, Davies DR, Friend PJ, Handa AI, et al. Subclinical rejection and borderline changes in early protocol biopsy specimens after renal transplantation. Transplantation. 2004. 77:1194–1198.
18. Heilman RL, Devarapalli Y, Chakkera HA, Mekeel KL, Moss AA, Mulligan DC, et al. Impact of subclinical inflammation on the development of interstitial fibrosis and tubular atrophy in kidney transplant recipients. Am J Transplant. 2010. 10:563–570.
19. Thierry A, Thervet E, Vuiblet V, Goujon JM, Machet MC, Noel LH, et al. Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. Am J Transplant. 2011. 11:2153–2161.
20. Henderson LK, Nankivell BJ, Chapman JR. Surveillance protocol kidney transplant biopsies: their evolving role in clinical practice. Am J Transplant. 2011. 11:1570–1575.
21. Rush D, Nickerson P, Gough J, McKenna R, Grimm P, Cheang M, et al. Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol. 1998. 9:2129–2134.
22. Gray D, Shepherd H, Daar A, Oliver DO, Morris PJ. Oral versus intravenous high-dose steroid treatment of renal allograft rejection. The big shot or not? Lancet. 1978. 1:117–118.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download