Abstract
A pancreatic hamartoma is a rare benign lesion that may be mistaken for malignancy. A pancreatic hamartoma can present with vague, non-specific symptoms, which can be difficult to diagnose despite modern diagnostic tools. We report here a pancreatic hamartoma diagnosed after surgical resection. A 52-year-old female presented with postprandial abdominal discomfort. Abdominal computed tomography and pancreatic magnetic resonance imaging revealed a 2.2 × 2.5-cm cystic mass in the pancreatic head. The patient underwent a pylorus-preserving pancreaticoduodenectomy. The histopathological and immunohistochemical studies helped make the diagnosis of pancreatic hamartoma. Here, we report a case of pancreatic hamartoma and review the relevant medical literature.
Hamartoma is defined as a benign (non-cancerous) tumor-like growth that consists of a disorganized mixture of cells and tissues that are normally found in the area of the body where the growth occurs. Hamartoma can arise in many different places and is most often asymptomatic and undetected unless seen on an image taken for another reason. The lung is the most common site for hamartoma. Pancreatic hamartoma is <1% of this type of tumor. To the best of our knowledge, fewer than 20 such cases have been reported in the literature. Pancreatic hamartoma is composed of three disarranged cellular components in varying proportions: acinar, islet, and ductal cells [1]. The pathogenesis of these tumors is still unknown. Here, we report a case of pancreatic hamartoma that was diagnosed by histopathological and immunohistochemical studies.
A 52-year-old woman presented with postprandial abdominal discomfort during the course of a month. Her past history was otherwise non-contributory. She was not alcoholic and had no history of pancreatitis. Physical examination revealed no characteristic features. Laboratory data, including amylase and lipase, were unremarkable. The level of carcinoembryonic antigen was elevated to 6.55 ng/mL (normal range, 0 to 4.7 ng/mL). The serum concentrations of carbohydrate antigen 19-9 and alfa-fetoprotein were within normal limits. Abdominal computed tomography (CT) revealed a nodule that measured 2.0 cm in maximum diameter in the head of the pancreas, which showed enhancement in the delayed phase (Fig. 1A). The gallbladder wall was thickened and enhanced after administering intravenous contrast medium (Fig. 1B). Pancreas magnetic resonance imaging (MRI) demonstrated a 2.2-cm, relatively well-defined, nodular mass in the pancreatic head (Fig. 2A-C). There was a 1.4-cm cystic or necrotic portion with septation in the superomedial aspect of the mass (Fig. 2D). No regional lymphadenopathy, ascites, or metastasis was demonstrated on MRI. The initial diagnosis was a solid pseudopapillary tumor or serous cystic neoplasm and adenomyomatosis of the gallbladder. The patient underwent pylorus-preserving pancreaticoduodenectomy.
In the operative field, we identified a 2.2 × 1.4-cm, well-circumscribed, non-encapsulated white to yellow hard mass that originated from the head of the pancreas. The cut surface of the tumor was whitish, and it showed signs of focal necrosis with cystic change (Fig. 3A).
Microscopically, the specimen showed haphazardly distributed cystic ductal elements lined by cuboidal to flattened epithelium, surrounded by well-differentiated acini embedded in fibro-inflammatory stroma (Fig. 3B, C). Immunohistochemical examination showed positive staining for CD34 (Fig. 3D) and synaptophysin, focal staining for CD56, and positive/negative staining for CD117 (c-kit) (Fig. 3E). There was no staining for chromogranin, Ki-67, desmin, or actin. Finally, the tumor was diagnosed as pancreatic hamartoma, and adenomyomasis of the gallbladder wall was confirmed.
The patient had an uneventful recovery, and she was discharged on postoperative day 32. There was no evidence of recurrence in the 10 months after surgery.
Pancreatic hamartoma is a nonneoplastic, mass-forming lesion of the pancreas and is extremely rare. Most cases reported in the literature as hamartoma appear to be examples of chronic pancreatitis in which pancreatic elements are haphazardly distributed in a hamartomatous fashion. Recently, true hamartomas of this organ have been characterized. Pancreatic hamartoma is divided into two subgroups: solid and cystic, and solid pancreatic hamartoma [2,3].
Table 1 summarizes the clinicopathological features of pancreatic hamartoma that have been reported in the literature. Pancreatic hamartoma can occur at any age (34 weeks to 62 years), but the average age of occurrence was 40 to 50 years (median, 46 years). The reported male-to-female ratio was 1:0.7. Eleven hamartomas were located in the head of the pancreas, four in the body or tail, one in the neck, and another occupied the entire pancreas. The average size of the mass was 4.32 cm (range, 0.9 to 11.5 cm) [1-10].
Pancreatic hamartoma is composed of three disarranged cellular components in varying proportions: acinar, islet, and ductal cells. But the pathogenesis of pancreatic hamartoma is still controversial. Although this tumor has been previously investigated by immunohistochemical, ultrastructural, and molecular biological studies, the precise mechanisms of its pathophysiology remain unknown.
The signs and symptoms are nonspecific. Most patients are asymptomatic or have vague symptoms (Table 1). Abdominal ultrasonography (US), CT, and MRI can be employed for preoperative diagnosis of hamartoma. US shows hyperechoic solid masses with or without a cystic change [6]. On CT, the tumor is a well-circumscribed, iso- or hypoattenuated, solid and/or cystic mass, with heterogeneous contrast enhancement [2,5,8]. MRI demonstrates a relatively well-defined mass. Although various imaging features can help, the diagnosis is often not direct. Pancreatic hamartoma can be difficult to diagnose preoperatively, and is usually diagnosed after pancreatectomy. In our case, the initial diagnosis was a solid pseudopapillary tumor or serous cystic neoplasm. However, the final diagnosis after surgical resection was pancreatic hamartoma. Thus, definitive diagnosis requires pathological examination of the excised lesion.
In pancreatic hamartoma, microscopic salient findings are a circumscribed lesion that is composed of a disorderly arrangement of well-differentiated endocrine and exocrine pancreatic tissue, and some cystically dilated ducts [2]. Immunohistochemically, the diagnosis of pancreatic hamartoma is supported by stromal cells that are positive for CD34 and/or CD117 (c-kit) [1,3]. CD34 is a myeloid stem-cell marker and is thought to play an important role in maintaining stromal integrity and inhibition of tumor cell migration. The fibroblasts in neoplastic and inflammatory pancreatic lesions have been described to express CD34 [10]. CD117 is a transmembrane tyrosine kinase receptor for stem cell factor and is encoded by the proto-oncogene c-kit [2]. c-kit mutations have been found in mast-cell tumors and gastrointestinal stromal tumor (GIST). In our case, the tumor was positive immunohistochemically for CD34, and positive/negative for c-kit. Thus, a diagnosis of GIST was excluded. The treatment of pancreatic hamartoma is surgical resection if possible.
In conclusion, diagnosis of pancreatic hamartoma is difficult preoperatively and is usually confirmed pathologically after surgery. Therefore, although extremely rare, pancreatic hamartoma should be taken into consideration as a differential diagnosis of a pancreatic tumor.
Figures and Tables
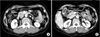 | Fig. 1(A) Abdominal computed tomography demonstrated a 2-cm nodule in the head of the pancreas. (B) The gallbladder wall was thickened and enhanced after administering intravenous contrast medium. |
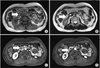 | Fig. 2(A-C) Pancreatic magnetic resonance imaging showed a 2.2-cm, relatively well-defined, nodular mass in the pancreatic head. (D) There was a 1.4-cm cystic or necrotic portion with septation in the superomedial aspect of the mass. |
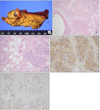 | Fig. 3(A) Gross and microscopic findings of pancreatic hamartoma. The cut surface of the tumor was whitish and showed signs of focal necrosis with cystic change. Histologically, the tumor was composed of haphazardly distributed, cystic ductal elements lined by cuboidal to flattened epithelium, surrounded by well-differentiated acini embedded in fibro-inflammatory stroma (B: H&E, ×20; C: H&E, ×200). The immunohistochemical findings showed positive staining for CD34 (D, ×100) and positive/negative staining for c-kit (E, ×100). |
References
1. Nagata S, Yamaguchi K, Inoue T, Yamaguchi H, Ito T, Gibo J, et al. Solid pancreatic hamartoma. Pathol Int. 2007. 57:276–280.
2. Pauser U, Kosmahl M, Kruslin B, Klimstra DS, Kloppel G. Pancreatic solid and cystic hamartoma in adults: characterization of a new tumorous lesion. Am J Surg Pathol. 2005. 29:797–800.
3. Pauser U, da Silva MT, Placke J, Klimstra DS, Kloppel G. Cellular hamartoma resembling gastrointestinal stromal tumor: a solid tumor of the pancreas expressing c-kit (CD117). Mod Pathol. 2005. 18:1211–1216.
4. Flaherty MJ, Benjamin DR. Multicystic pancreatic hamartoma: a distinctive lesion with immunohistochemical and ultrastructural study. Hum Pathol. 1992. 23:1309–1312.
5. Izbicki JR, Knoefel WT, Muller-Hocker J, Mandelkow HK. Pancreatic hamartoma: a benign tumor of the pancreas. Am J Gastroenterol. 1994. 89:1261–1262.
6. Wu SS, Vargas HI, French SW. Pancreatic hamartoma with Langerhans cell histiocytosis in a draining lymph node. Histopathology. 1998. 33:485–487.
7. McFaul CD, Vitone LJ, Campbell F, Azadeh B, Hughes ML, Garvey CJ, et al. Pancreatic hamartoma. Pancreatology. 2004. 4:533–537.
8. Thrall M, Jessurun J, Stelow EB, Adsay NV, Vickers SM, Whitson AK, et al. Multicystic adenomatoid hamartoma of the pancreas: a hitherto undescribed pancreatic tumor occurring in a 3-year-old boy. Pediatr Dev Pathol. 2008. 11:314–320.
9. Sampelean D, Adam M, Muntean V, Hanescu B, Domsa I. Pancreatic hamartoma and SAPHO syndrome: a case report. J Gastrointestin Liver Dis. 2009. 18:483–486.
10. Burt TB, Condon VR, Matlak ME. Fetal pancreatic hamartoma. Pediatr Radiol. 1983. 13:287–289.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download