Abstract
Purpose
Gastric surgery may potentiate delayed gastric emptying. Billroth I gastroduodenostomy using a circular stapler is the most preferable reconstruction method. The purpose of this study is to analyze the risk factors associated with delayed gastric emptying after radical subtotal gastrectomy with Billroth I anastomosis using a stapler for early gastric cancer.
Methods
Three hundred and seventy-eight patients who underwent circular stapled Billroth I gastroduodenostomy after subtotal gastrectomy due to early gastric cancer were analyzed retrospectively. One hundred and eighty-two patients had Billroth I anastomosis using a 25 mm diameter circular stapler, and 196 patients had anastomosis with a 28 or 29 mm diameter circular stapler. Clinicopathological features and postoperative outcomes were evaluated and compared between the two groups. Delayed gastric emptying was diagnosed by symptoms and simple abdomen X-ray with or without upper gastrointestinal series or endoscopy.
Results
Postoperative delayed gastric emptying was found in 12 (3.2%) of the 378 patients. Among all the variables, distal margin and circular stapler diameter were significantly different between the cases with delayed gastric emptying and no delayed gastric emptying. There were statistically significant differences in sex, body mass index, comorbidity, complication, and operation type according to circular stapler diameter. In both univariate and multivariate logistic regression analyses, only the stapler diameter was found to be a significant factor affecting delayed gastric emptying (P = 0.040).
Conclusion
In this study, the circular stapler diameter was one of the most significant predictable factors of delayed gastric emptying for Billroth I gastroduodenostomy. The use of a 28 or 29 mm diameter circular stapler rather than a 25 mm diameter stapler in stapled gastroduodenostomy for early gastric cancer can reduce postoperative delayed gastric emptying associated with anastomosic stenosis or edema with relative safety.
More than 100 years have passed since Billroth first described his procedure of reconstruction in 1881. Billroth I gastroduodenostomy has been the procedure of choice for distal gastrectomy. It provides more physiologic flow of food contents through the duodenum and decreases the possibility of metabolic problems and nutritional deficiency [1]. Since the introduction of surgical stapling devices, Ravitch and Steichen [2] reported his experiences of gastroduodenostomy using an end-to-end anastomosis (EEA) stapler in 1979, and Oka et al. [3] reported that gastroduodenostomy was performed using a double-stapling technique with EEA, which was separate from the anvil. Since then, Billroth I gastroduodenostomy with a circular stapler has become a popular method of anastomosis for gastric cancer, because it has several merits, including stability, simplicity, reduced operative time, etc.
Distal gastrectomy can lead to postgastrectomy syndromes such as dumping syndrome and reflux esophagitis, which are closely related to the rate of gastric emptying [4,5]. Prolonged gastric stasis after gastric surgery may occur occasionally, and most patients are able to eat a regular diet within 7 to 10 days after operation. The reported incidence of delayed gastric emptying (DGE) after gastrectomy has been reported to range from 5 to 30% [6-8].
Therefore, we evaluated DGE in patients who underwent radical subtotal gastrectomy with Billroth I gastroduodenostomy for early gastric cancer using a circular stapler. Also, we analyzed the predictable factors associated with DGE.
Patients with early gastric cancer treated with circular stapled Billroth I gastroduodenostomy between January 2003 and December 2008 were included in the present study. Among the 378 patients, laparoscopy assisted distal gastrectomy was performed in 264 cases, and conventional distal gastrectomy was performed in 114 cases. One hundred and eighty-two patients underwent Billroth-I anastomosis using a 25 mm diameter circular stapler, and 196 patients had anastomosis with a 28 or 29 mm diameter circular stapler. Clinicopathologic features such as age, gender, body mass index (BMI), comorbidity disease, tumor size, histologic type, tumor location, resection margin, tumor-node-metastasis stage, and postoperative outcomes were reviewed. DGE was diagnosed by patients' symptoms and simple abdomen X-ray with or without upper gastrointestinal series or endoscopy (Fig. 1).
All the values were expressed as means ± standard deviations (SDs). Postoperative follow-up periods were expressed as median ± SDs. Gastric cancer stage was classified according to the seventh edition of the American Joint Committee on Cancer staging criteria [9]. The patients enrolled in this study underwent standard D2 or above according to the 2010 Japanese gastric cancer treatment guidelines (ver. 3) [10].
Conventional distal gastrectomy with Billroth I gastroduodenostomy with lymphadenectomy was performed according to the 2010 Japanese gastric cancer treatment guidelines (ver. 3) [10]. Laparoscopy-assisted distal gastrectomies were performed according to the standard procedure guidelines as described in a previous report [11]. In the early period of laparoscopy assisted distal gastrectomy, we applied a 25 mm diameter circular stapler to the Billroth I gastroduodenostomy, because a 25 mm diameter circular stapler was suitable forsmall wounds. After gaining experience with laparoscopy assisted distal gastrectomy, we applied a 28 or 29 mm diameter circular stapler to the Billroth I gastroduodenostomy even if the wound size was small. In contrast, we used a 28 or 29 mm diameter circular stapler during the Billroth I gastroduodenostomy of conventional distal gastrectomy from the beginning.
Clinical characteristics of patients were summarized as a whole, as well as described specifically for subgroups by descriptive statistics. After descriptive analyses were performed, a Fisher's exact test was used to compare categorical variables between groups, while a Student's t-test was used to compare continuous variables between groups.
Odds ratio (OR) for comparison of the two groups was summarized with its 95% confidence interval (CI) and P-value using logistic regression. The multivariate model was created using a backward elimination method, and the probability was set at 0.20 for removal. ORs were also adjusted for factors affecting the response variable. P-values lower than 0.05 were considered statistically significant. All statistical analyses were carried out using PASW ver. 18.0 (IBM Co., Armonk, NY, USA).
Among the 378 patients, postoperative DGE was found in 12 patients (3.2%). Clinicopathological and postoperative outcomes for the 378 patients with regard to the presence of DGE are shown in Table 1. There were statistically significant differences in distal margin and circular stapler diameter between the groups (P = 0.045 and P = 0.017, respectively). We also investigated the clinicopathologic and postoperative outcomes according to the circular stapler diameter between the 25 mm diameter stapler and 28 or 29 mm diameter stapler. There were statistically significant differences in gender, BMI, presence of comorbidity, operation method, operation time, presence of complication, and presence of DGE between the two sizes of stapler (Table 2).
In 10 out of 12 patients who developed DGE, circular staples with 25 mm diameter were used. Another one with 28 mm, and 1 with 29 mm developed DGE. Mean postoperative day that started the findings of DGE was 21.6 days, and most cases were diagnosed by simple abdominal X-rays or esophagogastroduodenoscopy. Among the 12 cases, 2 had anastomotic stenosis, 8 had anastomotic edemaand the remaining 2 had gastric atony. Ten patients were managed with conservative treatment, but the 2 underwent endoscopic balloon dilatation for anastomotic narrowing or stricture (Table 3).
In univariate analyses, BMI and circular stapler diameter were found to be significant factors affecting DGE (OR, 0.25; 95% CI, 0.07 to 0.94; P = 0.040; OR, 5.64; 95% CI, 1.22 to 26.10; P = 0.027, respectively). The circular stapler diameter remained significantly associated with DGE based on multivariate analysis (OR, 5.16; 95% CI, 1.11 to 24.02; P = 0.037) (Table 4).
DGE is considered to be a postgastrectomy syndrome. Its occurrence in the early postoperative period is generally thought to spontaneously resolve within 6 weeks of surgery, and the temptation to reoperate on a non-obstructive stomach should be avoided [8,12]. There are various definitions of DGE in the literature. Cohen and Ottinger [6] stated that DGE was a condition in which patients are unable to eat a solid diet after 2 postoperative weeks. Bar-Natan et al. [8] defined DGE as the inability to eat a regular diet after 10 postoperative days. In our study, the time in which DGE occurred was different case by case, and we defined DGE by patients' symptoms of gastric fullness, nausea, vomiting, and simple abdomen X-ray with or without upper gastrointestinal series or endoscopy.
We analyzed the predictable factors associated with DGE with Billroth I gastroduodenostomy using a circular stapler for early gastric cancer. Although there was statistically significant difference in the distal margin between the DGE group (12 patients) and non-DGE group (366 patients), we found that the circular stapler diameter was a more significant factor affecting DGE. In addition, there were statistically significant differences according to circular stapler diameter with respect to BMI, operation method, operation time, and the presence of comorbidity, complication, and DGE. However, this result can be explained by the fact that, in the early period of performing laparoscopic gastrectomy, we selected patients with lower BMI and no comorbidity to ensure a favorable performance of laparoscopy assisted distal gastrectomy; in these patients, laparoscopy assisted distal gastrectomy using a 25 mm diameter circular stapler was more commonly performed. Laparoscopic gastrectomy required a longer operation time than conventional distal gastrectomy, the operation time was statistically longer in the 25 mm group than in the 28 or 29 mm group because more cases of laparoscopic gastrectomy were performed in the 25 mm circular stapler group than in the 28 or 29 mm circular stapler group.
There were several causes of DGE. First, the underlying diseases of patients, particularly diabetes and malnutrition, emerged as preoperative risk factors for postoperative gastric stasis [8]. Some reports have described an association between insulin-dependent diabetes and postoperative motility problems [13,14]. In our series, DGE more commonly occurred in the 25 mm group, despite the fact that the incidence of comorbidity was lower in the 25 mm group (47 patients, 25.8%) than in the 28 or 29 mm group (74 patients, 37.8%). Also, among the 378 patients, there were 42 patients with diabetes; 3 of those patients developed DGE. We then performed statistical analysis of DGE of the 42 diabetes patients. However, there was no statistically significant difference with respect to DGE because the number of diabetic patients who had DGE (3 patients) was too small. Malnutrition also correlates with the development of postoperative gastric stasis. However, the majority of patients in our study were incidentally detected with early gastric cancer during regular individual checkups, and their nutritional status was adequate.
Second, other causes of DGE were anastomosis narrowing due to edema or stenosis. Many potential contributing factors to the etiology of anastomotic stenosis with a circular stapler have been proposed. These include tension on the anastomosis, local tissue ischemia, subclinical leak, injury from acid exposure, and submucosal hematoma created during suturing [15,16]. Fisher et al. [17] and Gould et al. [18] reported the risk factor of gastrojejunostomy stenosis according to circular stapler diameter for laparoscopic Roux-en-Y gastric bypass in morbid obesity. They used 21 mm and 25 mm diameter circular staplers for gastrojejunostomy. They showed that the 21 mm diameter circular stapler resulted in more stenosis and needed additional endoscopic balloon dilatation. In our study, there were more incidences of DGE in the 25 mm group than in the 28 or 29 mm group. Therefore, we could confirm that circular stapler diameter was the only risk factor of DGE in our univariate and multivariate analysis.
Third, DGE may result from truncal vagotomy as a result of denervation of the stomach for gastrectomy [19,20]. During conventional radical subtotal gastrectomy, lymph nodes and vagal nerves are removed around the esophagogastric junction area. Such a procedure of denervation of the stomach results in loss of gastric compliance. However, in our series, all patients underwent truncal vagotomy for clear dissection of lymph nodes of the esophagogastric junction area. Since vagotomy was performed on all the patients in our series, it could be excluded from the statistical factors affecting DGE.
Several solutions are available for DGE. First, traditional medical therapy consists of behavior and diet modification, nasogastric tube suction, and the use of prokinetic drugs such as bethanechol, metoclopramide, erythromycin, and more recently, cisapride. Dietary measures and prokinetic drugs bring symptomatic relief in most patients. Some patients with severe nausea and vomiting will require antiemetic medications. Second, endoscopic or radiologic dilation of anastomotic stenosis can be performed when anastomotic edema or stenosis does occur. There have been many reports of endoscopic balloon dilation with gastric bypass surgery [21-24]. Endoscopic balloon dilation of the strictured anastomosis is a reliable and safe treatment and has less morbidity than surgical revision. At present, it is the standard procedure for managing such the complication of anastomotic stenosis.
In our study of 12 patients with DGE, 10 patients were treated by conservative management such as diet modification, nasogastric tube suction, and the use of prokinetic drugs. Two patients did not improve in spite of conservative management. We evaluated the cause of DGE in two patients after massive nasogastric tube irrigation. On the endoscopic findings, there was stenosis at the anastomosis site. They were successfully treated by endoscopic balloon dilatation. Since that time, there has been no additional endoscopic intervention necessary.
In order to prevent anastomotic stenosis, circular staplers with diameters as large as possible would be recommended, but it should be taken into account that the large diameter staples may result in postoperative bile reflux and subsequent gastritis. A recent report introduced that in a group using 25 mm circular staples stasis developed in the early postoperative period, but in the later stage it showed no difference; while that with 29 mm circular staples it showed gastritis and bile reflux more frequently than the other group [25]. It is necessary that more investigation about the incidences of gastritis and bile reflux following circular staples and their prevention and management should be preceded in our study.
The drawbacks of this study include the retrospective design of a small number of cases and the possibility of bias in data. In fact, the number of the patients enrolled in this study was too small to assert the causes of DGE after gastrectomy. Therefore, a prospective, randomized, controlled trial with available indications will be essential to overcome those drawbacks.
However, we revealed that the circular stapler diameter was one of the most significant predictable factors of DGE for Billroth I gastroduodenostomy. The use of proper circular stapler diameter was mandatory, and DGE was well treated by conservative or endoscopic intervention after Billroth I gastroduodenostomy.
In conclusion, we demonstrated that circular stapler diameter was the most important risk factor of DGE for Billroth I gastroduodenostomy. We also recommend that the use of a 28 or 29 mm diameter circular stapler for Billroth I gastroduodenostomy is more suitable than the use of a 25 mm diameter circular stapler to reduce the DGE associated with anastomotic stenosis or edema.
Figures and Tables
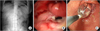 | Fig. 1Radiologic and endoscopic finding of delayed gastric emptying. (A) Simple abdomen X-ray shows dilated stomach with food material. (B) Severe stenosis of anastomosis site after Billroth I gastroduodenostomy. Opening is seen at inferior direction of anastomosis site. Opening was too small for endoscope to pass through. Ulcer lesion is seen below anastomotic site. (C) Endoscopic view of gastroduodenostomy stenosis undergoing balloon dilatation. Luminal narrowing is seen due to anastomotic stenosis. Balloon dilatation by 20→25→30 psi was done for 2 minutes. There developed no complication such as bleeding due to procedure. Widening of stenosis site can be seen. |
Table 1
Clinicopathological and postoperative outcomes according to presence of delayed gastric emptying

References
1. Beyan C, Beyan E, Kaptan K, Ifran A, Uzar AI. Post-gastrectomy anemia: evaluation of 72 cases with post-gastrectomy anemia. Hematology. 2007. 12:81–84.
2. Ravitch MM, Steichen FM. A stapling instrument for end-to-end inverting anastomoses in the gastrointestinal tract. Ann Surg. 1979. 189:791–797.
3. Oka M, Maeda Y, Ueno T, Iizuka N, Abe T, Yamamoto K, et al. A hemi-double stapling method to create the Billroth-I anastomosis using a detachable device. J Am Coll Surg. 1995. 181:366–368.
4. Hulme Moir I. The role of altered gastric emptying in the initiation of clinical dumping. Scand J Gastroenterol. 1979. 14:463–467.
5. Fujiwara Y, Nakagawa K, Tanaka T, Utsunomiya J. Relationship between gastroesophageal reflux and gastric emptying after distal gastrectomy. Am J Gastroenterol. 1996. 91:75–79.
6. Cohen AM, Ottinger LW. Delayed gastric emptying following gastrectomy. Ann Surg. 1976. 184:689–696.
7. Jordon GL Jr, Walker LL. Severe problems with gastric emptying after gastric surgery. Ann Surg. 1973. 177:660–668.
8. Bar-Natan M, Larson GM, Stephens G, Massey T. Delayed gastric emptying after gastric surgery. Am J Surg. 1996. 172:24–28.
9. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 2010. 7th ed. New York: Springer.
10. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011. 14:113–123.
11. Kim MC, Choi HJ, Jung GJ, Kim HH. Techniques and complications of laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer. Eur J Surg Oncol. 2007. 33:700–705.
12. Behrns KE, Sarr MG. Diagnosis and management of gastric emptying disorders. Adv Surg. 1994. 27:233–255.
13. Smale BF, Copeland JG, Reber HA. Delayed gastric emptying after operation for obstructing peptic ulcer disease: the influence of cimetidine. Surgery. 1984. 96:592–598.
14. Hom S, Sarr MG, Kelly KA, Hench V. Postoperative gastric atony after vagotomy for obstructing peptic ulcer. Am J Surg. 1989. 157:282–286.
15. Sanyal AJ, Sugerman HJ, Kellum JM, Engle KM, Wolfe L. Stomal complications of gastric bypass: incidence and outcome of therapy. Am J Gastroenterol. 1992. 87:1165–1169.
16. Wolper JC, Messmer JM, Turner MA, Sugerman HJ. Endoscopic dilation of late stomal stenosis. Its use following gastric surgery for morbid obesity. Arch Surg. 1984. 119:836–837.
17. Fisher BL, Atkinson JD, Cottam D. Incidence of gastroenterostomy stenosis in laparoscopic Roux-en-Y gastric bypass using 21- or 25-mm circular stapler: a randomized prospective blinded study. Surg Obes Relat Dis. 2007. 3:176–179.
18. Gould JC, Garren M, Boll V, Starling J. The impact of circular stapler diameter on the incidence of gastrojejunostomy stenosis and weight loss following laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2006. 20:1017–1020.
19. Yamagishi T, Debas HT. Control of gastric emptying: interaction of the vagus and pyloric antrum. Ann Surg. 1978. 187:91–94.
20. Mayer EA, Thomson JB, Jehn D, Reedy T, Elashoff J, Deveny C, et al. Gastric emptying and sieving of solid food and pancreatic and biliary secretions after solid meals in patients with nonresective ulcer surgery. Gastroenterology. 1984. 87:1264–1271.
21. Barba CA, Butensky MS, Lorenzo M, Newman R. Endoscopic dilation of gastroesophageal anastomosis stricture after gastric bypass. Surg Endosc. 2003. 17:416–420.
22. Ahmad J, Martin J, Ikramuddin S, Schauer P, Slivka A. Endoscopic balloon dilation of gastroenteric anastomotic stricture after laparoscopic gastric bypass. Endoscopy. 2003. 35:725–728.
23. Huang CS, Forse RA, Jacobson BC, Farraye FA. Endoscopic findings and their clinical correlations in patients with symptoms after gastric bypass surgery. Gastrointest Endosc. 2003. 58:859–866.
24. Go MR, Muscarella P 2nd, Needleman BJ, Cook CH, Melvin WS. Endoscopic management of stomal stenosis after Roux-en-Y gastric bypass. Surg Endosc. 2004. 18:56–59.
25. Kim MK, Park JM, Choi YS, Chi KC. Smaller-diameter circular stapler has an advantage in Billroth I stapled anastomosis after laparoscopy-assisted distal gastrectomy. J Laparoendosc Adv Surg Tech A. 2012. 22:236–241.




 ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download