Abstract
The generally accepted treatment for infected aortic aneurysms involves open surgical resection and debridement, with in situ or extra-anatomical bypass. Occasionally, endovascular management can be substituted for the standard operation dependent on the patient's condition. We report the case of an 81-year-old female with a ruptured infected aortic aneurysm and sepsis, successfully treated endovascularly. She had been on oral antibiotics for one year and is doing well 2 years after discharge.
A ruptured infected aortic aneurysm with staphylococcal sepsis is rare yet potentially life-threatening condition. Although ruptured infected aneurysm is usually treated surgically, it has still high operative mortality and morbidity, especially high surgical risk. Despite recent improvement in endovascular treatment of abdominal aortic aneurysm and antibiotic therapy, a ruptured infected aortic aneurysm coexisting with sepsis is still a lethal condition. We report one case that in a highly risk patient endovascular approach with prolonged antibiotic therapy was one of options for successful results.
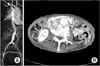
A 81-year-old female with history of hypertension was admitted to the hospital with abdominal pain, anorexia, and mild fever for two months' duration. She was admitted to the gastroenterology for gastritis two months ago and at that time she was diagnosed as infectious colitis and her blood culture yielded staphylococcus aureus, which was treated with intravenous administration with ceftriaxone. The computed tomography (CT) of her abdomen at that time showed a normal abdominal aorta (Fig. 1A).
On admission, the patient looked unwell, and had a temperature of 37.7℃, pulse rate of 72 beats per minute, respiration rate of 20 per minute, and blood pressure of 150/70 mmHg. Her heart rate was regular, without murmur, rub, or gallop. Her lungs were clear without rales, rhonchi, wheezes, or crackles. The abdomen was soft but tender on mid abdomen with pulsating mass. The upper extremities were neurologically normal. She was bed-ridden state. A CT image of abdominal aorta demonstrated irregular aneurysmal dilatation (maximum 6 cm) of the abdominal aorta at the L3-L4 level, along with out-pouching area and soft tissue masses that showed heterogeneous enhancement. Enhancing and nonenhancing elements were seen within the dilated aortic lumen, along with a crescent-shaped low-signal area outside the aorta, suggesting leakage of the aneurysm (Fig. 1B). She had a total white blood count of 20,600/mm3, with a differential count of 94% neutrophils, 3% lymphocytes and 3% monocytes, and a C-reactive protein of 30.67 mg/dL. The blood culture showed the growth of methicillin resistant staphylococcus aureus. Preoperative transthoracic echocardiography showed no vegetation on the valve and preserved heart function. The patient and her relatives were refused the open resection.
The patient was prepared for surgery on an urgent basis and underwent endovascular surgery in a standard operating room equipped with a mobile C-arm and image intensifier. During the procedure, the patient had permissive hypothermia and was heparizied with 3,000 U heparin. Both groins were exposed, diagnostic angiography catheter was introduced via the left common femoral artery for intraoperative imaging. The ruptured aorta was then covered with Edurant aorto-uni-iliac graft (Medtronic Inc., Mineapolis, MN, USA) via the right common femoral artery. A 10-mm Amplatzer (St. Jude Medical, St. Paul, MN, USA) vascular plug was placed in the left common iliac artery. Completion angiography showed a successful exclusion of the ruptured aortic segment without any signs of endoleak. And then fem-fem bypass was done with 8 mm polytetrafluoroethylene graft to restore blood flow to the left leg (Fig. 2A).
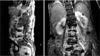
The patient had an uneventful postoperative recovery without complications, but constantly complained of back pain, which was revealed spondylitis at the level of aneurysm rupture area (Fig. 3). Antibiotic treatment with intravenous vancomycin infusion was continued for eight weeks. And then the blood culture had no growth and the C-reactive protein concentration returned to normal at discharge. Three-month follow-up CT showed near absorption of the periaortic hematoma (Fig. 2B). The patient had been on oral antibiotics for one year and doing well at 2 years after discharge.
Mycotic aortic aneurysms are quite infrequent, 1 to 1.8% of aortic aneurysms [1]. Nearly 50% are seen at the infrarenal aorta, and 25% is involved the juxtarenal or pararenal aorta [2]. Despite advances in antibiotic treatment, pure medical management for mycotic aneurysms is often inadequate, and gold standard therapy remains surgical resection, debridement of the infected aorta and the surrounding tissue, and either an in situ interposition or extra-anatomical bypass [3]. However, these patients have high surgical risk and mortality, especially in non-Salmonella infection and advanced age [4]. Fillmore and Valentine [5] determined that sepsis is the leading cause of death for surgical infected aneurysm patients. Five independent variables are known to associate with operative mortality: extensive peri-aortic infection, female sex, Staphylococcus aureus infection, aneurysm rupture and supra-renal location [6]. The negative effect of rupture on mortality is huge that the mortality rate of 13% with intact aneurysm increased to 33.3% in case of rupture.
Endovascular repair of atherosclerotic thoracic and abdominal aneurysms is well established. However, placing an endovascular graft in an infected area remains counter-intuitive and against general surgical principles. Semba et al. [7] first proposed endovascular aortic repair (EVAR) as an alternative approach. A literature review about endoluminal management of mycotic aneurysm revealed that EVAR seemed a possible alternative method for treating mycotic aneurysm and the only significant independent risk factors for persistent infection were rupture of aneurysm and fever [8]. If the fever persists after EVAR, a definite surgical treatment should be considered. EVAR has significant advantages over surgical resection as it avoids a large incision, full heparinization, extracorporeal circulation, aortic cross-clamping, interference with respiratory function, and the need for massive transfusion.
Age is a predictor of poor outcome with major surgery, with one report finding that individuals of 80 years old compared with patients 65 to 69 had a twofold higher operative mortality [9]. The perioperative mortality in EVAR ranges from 1.9 to 5%; however, perioperative mortality in open procedures ranges from 5 to 10%. For octogenarians, one of the most important factors in recovery is rapid return to baseline and prompt discharge from hospital. EVAR had a statistically significant more rapid return to ambulation than the open repair. Thirty-six percentage of individuals who underwent open repair of abdominal aortic aneurysm did not return to be ambulatory (preoperatively 100% ambulatory) [10].
Antibiotic treatment of infected aneurysms ranges anywhere from a few weeks to lifelong after surgery. And the regimens should be based on cultures and sensitivities when a pathogen can be isolated. If no pathogen is identified, the regimens should be broad, covering G(+), G(-), anaerobic and fungus.
Figures and Tables
 | Fig. 1(A) Contrast-enhanced computed tomography images revealed near normal abdominal aorta two months before admission and (B) ruptured abdominal aortic aneurysm at admission. |
References
1. Svensson LG, Crawford ES. Aortic dissection and aortic aneurysm surgery: clinical observations, experimental investigations, and statistical analyses. Part I. Curr Probl Surg. 1992. 29:817–911.
2. Miller DV, Oderich GS, Aubry MC, Panneton JM, Edwards WD. Surgical pathology of infected aneurysms of the descending thoracic and abdominal aorta: clinicopathologic correlations in 29 cases (1976 to 1999). Hum Pathol. 2004. 35:1112–1120.
3. Müller BT, Wegener OR, Grabitz K, Pillny M, Thomas L, Sandmann W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001. 33:106–113.
4. Hsu RB, Chen RJ, Wang SS, Chu SH. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg. 2004. 40:30–35.
5. Fillmore AJ, Valentine RJ. Surgical mortality in patients with infected aortic aneurysms. J Am Coll Surg. 2003. 196:435–441.
6. Oderich GS, Panneton JM, Bower TC, Cherry KJ Jr, Rowland CM, Noel AA, et al. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001. 34:900–908.
7. Semba CP, Sakai T, Slonim SM, Razavi MK, Kee ST, Jorgensen MJ, et al. Mycotic aneurysms of the thoracic aorta: repair with use of endovascular stent-grafts. J Vasc Interv Radiol. 1998. 9(1 Pt 1):33–40.
8. Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg. 2007. 46:906–912.
9. Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Eff Clin Pract. 2001. 4:172–177.
10. Williamson WK, Nicoloff AD, Taylor LM Jr, Moneta GL, Landry GJ, Porter JM. Functional outcome after open repair of abdominal aortic aneurysm. J Vasc Surg. 2001. 33:913–920.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download