Abstract
We describe two patients, with no previous history of vascular problems but poor lung function, who experienced septic shock due to bowel ischemia. Both were fed an enteral formula rich in fiber using a feeding tube and experienced septic shock with regular enteral feeding. Surgical finding showed hemorrhagic ischemia in the bowel. The pathologic finding suggests these changes may have been due to inspissations of bowel contents, which may put direct pressure on the mucosa of the bowel wall, leading to local impairment of mucosal and submucosal blood flow with subsequent bowel necrosis. Bowel ischemia may have been precipitated by an increased mesenteric blood flow requirement in combination with a metabolically stressed bowel. Patients in the intensive care unit fed a fiber-rich enteral formula may have inspissated bowel contents, leading to bowel ischemia, suggesting that the use of fiber-rich formula should be limited in patients at high-risk of bowel ischemia.
Enteral nutrition is the preferred method to provide nutritional support in critically ill patients [1]. Despite the advantages of enteral feeding, enteral nutrition may not be tolerated by all patients and may cause complications such as diarrhea, constipation, aspiration and bowel ischemia. We describe two patients who manifested fatal complication of bowel ischemia associated with enteral feeding.
A 53-year-old man with hypertension and diabetes mellitus, who was a Hepatitis B virus carrier visited the hospital due to abdominal discomfort. Computed tomography scanning showed a liver mass. Upon opening his abdomen for mass resection, we found that the lesions were multiple and not resectable. We therefore performed radiofrequency ablation (RFA) in the operating room. There were no abnormal findings in the small or large intestine.
Two days after RFA, he was transferred to the intensive care unit (ICU) due to sepsis with acute respiratory distress syndrome, probably caused by RFA-related liver abscess. He had improved for 2 weeks with positive pressure ventilation. During recovery phase, he continued to receive 30 kcal/kg of enteral nutrition via a feeding tube with regular bowel movement.
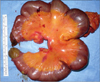
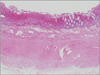
On ICU 15th day, however, we observed new onset leukocytosis, tachycardia and fever. Moreover, his fever became worse and his intra-abdominal pressure increased to up to 17 mmHg. The next day, he showed unstable vital signs, with a sudden drop of blood pressure. Cultures of his blood, catheter tip, and ascites samples revealed no infection. After excluding other causes of septic shock, we began to suspect that it was of bowel origin. During exploration of his abdomen, we observed mucosal infarction and congestion of the entire intestine, which was filled with mud-like contents (Fig. 1). We therefore performed a total colectomy and small bowel resection for bowel necrosis, but the patient died of hepatic failure soon afterward. After operation, we can confirm pathologic findings of specimen, we couldn't find an evidence of acute vessel obstruction (Fig. 2).
A 77-year-old man, who had been diagnosed with chronic obstructive pulmonary disease (COPD) on both lower lungs with underlying emphysema, visited Asan Medical Center with mid common bile duct cancer that had been diagnosed at another hospital. After we performed a bile duct resection and cholecystectomy, he was admitted to the ICU for pneumonia caused by exacerbation of his COPD. He received mechanical ventilator support for 7 days. With recovery of his condition, he was administered enteral nutrition, consisting of a 25 kcal/kg of formula rich in dietary fiber via a feeding tube for 5 days. On ICU 10th day, however, he experienced septic shock, with tachycardia, fever and a stuporous mental state. His blood pressure decreased despite receiving vasopressor and inotropic agents.
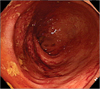
We hypothesized that the bowel ischemia of this patient was associated with enteral nutrition due to abdominal tenderness and a rectum filled with mud-like stool even with regular bowel movement. A sigmoidofiberscopy showed increased vascularity of the rectal mucosa, as well as increased bowel edema, hyperemia and submucosal hemorrhage (Fig. 3).
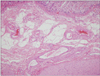
He underwent an emergency Hartmann's operation, resulting in progressive improvement in his general condition. After surgery, he was transferred to general ward on ICU 21 days. Pathologic finding of the resected bowel specimen didn't show the evidence of vessel injury or ischemia (Fig. 4). However, the finding of thinning and diffuse flattening with hemorrhagic necrosis of mucosal layer and congestion of submucosa is suggesting hemorrhagic infarction caused by direct pressure of mucosal wall.
Enteral nutrition is regarded as a preferred route for nutrition support of the critically ill patient with functioning digestive tract. Enterally administrated nutritional support has been found to prevent mucosal weight loss, to increase endothelial proliferation, and to improve the maintenance of gut mucosal integrity [2], with maintenance of trophism apparently being the key to the prevention of bacterial translocation [3]. In addition, because the gut is the largest producer of humoral antibodies in the body, it is believed that improved immune responses to bacterial challenge in enterally, compared with parenterally, fed individuals may be important in preventing septic complications [2].
Despite these advantages, enteral nutrition may not be tolerated by all patients and may cause fatal complications such as diarrhea, constipation, aspiration and bowel ischemia [4]. Especially, nasoenteric feeding in critically ill patients is often complicated by diarrhea. When diarrhea develops in critically ill patients, it adversely affects their nutritional status, electrolyte imbalance and nursing care and places them at increased risk for infection in the recovery phase. This results in longer hospital stay, higher medical expenses and increased mortality. Attempts to control the diarrhea that accompanies tube feeding include either changing the enteral formula which is containing fiber.
We experienced two patients who manifested bowel ischemia associated with enteral nutrition. These two patients described here have several features in common. The nutritional formulas they were fed contained plenty of fiber, they received postoperative mechanical ventilatory support due to poor lung function, and they have no underling vascular disease which cause acute bowel ischemia. In both patients, however, inspissated muddy stools were seen in the resected bowel specimen. Several mechanisms have been proposed as contributing towards the development of enteral tube feeding associated diarrhea, which are discussed below.
First, the inspissated bowel contents may have put direct pressure on the mucosa of the bowel wall, leading to local impairment of mucosal blood flow with subsequent ischemic changes in the bowel. Both patients were supplied dietary fiber daily for several days. This commercially available formula (Jevity), contains soy polysaccharide, which has up to 94% insoluble dietary fiber with no soluble dietary fiber. Dietary fiber has become a standard component of enteral nutrition formulas to prevent and treat chronic constipation [5]. Dietary fiber increases intestinal transit and stool bulk, stimulating voluntary defecation. The increase in stool bulk is thought to result from the residual, unfermented fiber, as well as from water held by gel formed by unfermented fibers. Direct pressure leading to bowel ischemia was suggested by our surgical findings. Pathologic examination also showed the evidence of mechanism of bowel ischemia. Pathologic finding showed no acute vessel occlusion, atherosclerosis of arterial vessels, or thrombosis. Mucosal thinning and diffuse flattening with hemorrhagic infarction are indicators of direct pressure from the lumen. Generally, in critically ill patient who have risks of bowel ischemia, most guideline recommend to supply soluble fiber-rich enteral formula.
Next, increased energy demand from enterocytes may also have contributed to enteral nutrition-associated bowel ischemia, in association with ischemic bowel change [6], resulting in mismatches in the oxygen demand to supply ratio in the intestinal mucosa. Enteral nutrition leads to tropism of enterocytes [7]. In the presence of luminal nutrients, splanchnic blood flow increases up to 200% that of baseline in response to the augmented demand of the intestinal mucosa, resulting in a regional blood-flow redistribution known as postprandial hyperemia [8].
In conclusion, enteral nutrition has many benefits for critically ill patients and dietary fiber is important for stimulating the structural and functional trophism of the mucosa, preventing diarrhea by absorption of sodium and water [9] and preventing bacterial translocation [10]. However, the contents of enteral formula may lead to complications. An overabundance of insoluble dietary fiber in enteral nutrition may aggravate bowel ischemia. Stool bulkiness resulting from insoluble dietary fiber and low transit time may result in inspissated bowel contents. Especially, in patients who are hemodynamically unstable and hypoxic, bowel ischemia could also exacerbated. In these situations, it is important to reduce the proportion of insoluble dietary fiber. Physician in ICUs should be aware of enteral nutirtion associated non-occlusive bowel ischemia in patients with fever, tachycardia, leukocytosis, and septic shock of unknown origin.
Figures and Tables
References
1. Munshi IA, Steingrub JS, Wolpert L. Small bowel necrosis associated with early postoperative jejunal tube feeding in a trauma patient. J Trauma. 2000. 49:163–165.
2. Minard G, Kudsk KA. Is early feeding beneficial? How early is early? New Horiz. 1994. 2:156–163.
3. Alexander JW. Nutrition and translocation. JPEN J Parenter Enteral Nutr. 1990. 14:5 Suppl. 170S–174S.
4. Adam S, Batson S. A study of problems associated with the delivery of enteral feed in critically ill patients in five ICUs in the UK. Intensive Care Med. 1997. 23:261–266.
5. Tramonte SM, Brand MB, Mulrow CD, Amato MG, O'Keefe ME, Ramirez G. The treatment of chronic constipation in adults. A systematic review. J Gen Intern Med. 1997. 12:15–24.
6. Lee JS, Auyeung TW. A comparison of two feeding methods in the alleviation of diarrhoea in older tube-fed patients: a randomised controlled trial. Age Ageing. 2003. 32:388–393.
7. Bohlen HG. Intestinal tissue PO2 and microvascular responses during glucose exposure. Am J Physiol. 1980. 238:H164–H171.
8. Serpa LF, Kimura M, Faintuch J, Ceconello I. Effects of continuous versus bolus infusion of enteral nutrition in critical patients. Rev Hosp Clin Fac Med Sao Paulo. 2003. 58:9–14.
9. Emery EA, Ahmad S, Koethe JD, Skipper A, Perlmutter S, Paskin DL. Banana flakes control diarrhea in enterally fed patients. Nutr Clin Pract. 1997. 12:72–75.
10. Mosenthal AC, Xu D, Deitch EA. Elemental and intravenous total parenteral nutrition diet-induced gut barrier failure is intestinal site specific and can be prevented by feeding nonfermentable fiber. Crit Care Med. 2002. 30:396–402.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download