Abstract
Purpose
Clostridium difficile colitis (CDC) is a nosocomial infection. We attempted to discover the risk factors for the development of CDC in patients admitted to our surgical ward.
Methods
We conducted a retrospective chart review of all patients admitted to our surgical ward between January 2010 and July 2011. CDC was confirmed when toxin A/B or toxin B polymerase chain reaction was detected in the stool and clinical symptoms, such as diarrhea, were present. We divided patients into the CDC and non-CDC groups, and compared the clinical features between the two groups.
Results
The rate of CDC occurrence
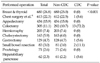
was 0.4% (19/4,720 patients). Univariate analysis showed that colectomy (P < 0.001), hospital stays longer than 10 days (P < 0.001), aged over 55 years (P < 0.001) and transfer from medical ward (P = 0.009) were significant parameters for CDC. Multivariate analysis showed that colectomy (P < 0.001; odds ratio [OR], 8.405; 95% confidence interval [CI], 2.927 to 24.132) and hospital stays longer than 10 days (P = 0.035; OR, 10.253; 95% CI, 1.176 to 89.392) were high risk factors for CDC occurrence in the surgical ward.
Clostridium difficile colitis (CDC) is a nosocomial infection caused by toxins produced by unopposed proliferation of the bacterium C. difficile [1]. Antibiotic therapy alters the colonic flora and allows C. difficile to flourish. All inpatients who have received antibiotics are at risk of C. difficile infection. The infection route of C. difficile is fecal to oral. Outside of the surgical ward, the C. difficile bacterium can persist in the form of heat-resistant spore for months or years [2]. Therefore, environmental contamination with C. difficile spores in hospitals increases the incidence of CDC.
During the early and mid 2000s, the reported incidence, severity and mortality of C. difficile infection increased in the United States. This increase may be due to the emergence of virulent strains [3]. Although a previous study reported that the incidence of CDC has increased in Korea, it is not certain whether or not the cause is due to new strains [4]. It has been demonstrated that increasing use of antibiotics is a reason of an increased incidence of CDC in Korea [5]. Not all patients who are exposed to C. difficile spores do develop CDC [6]. We attempted to find out the risk factors for the development of CDC in patients admitted to the surgical ward. We hypothesized that specific risk factors for CDC might be in the surgical ward.
We conducted a retrospective chart review of all patients admitted to surgical ward of Hallym University Sacred Heart Hospital between January 2010 and July 2011. The surgical ward is composed of 1 nursing station and 80 beds on 1 floor. The surgical ward was mostly occupied by patients who needed abdominal and thoracic surgeries. If some beds are unoccupied, patients from other departments are also admitted.
We divided patients into the CDC and non-CDC groups. We compared age, sex, admission duration, primary disease, leukopenia and whether or not the patient was transferred from the medical ward between the 2 groups.
CDC was confirmed when toxin A/B or toxin B gene was detected in the stool and clinical symptoms, such as diarrhea, were present. Toxin A/B were examined using enzyme-linked fluorescent immunoassay (VIDAS, bio-Mérieux sa, l'Etoile, France) and B gene were detected using the polymerase chain reaction (PCR) assays (Master Cycler, eppendorf, Hamburg, Germany) for B genes in C. difficile isolates that grew from the stool cultures. The instruments for the toxin A/B and PCR assays were not changed from 2006 to 2011. When the test for toxin A/B was equivocal but clinical symptoms were present, the patient was also classified as part of the CDC group. The non-CDC group was defined as a group of patients who did not have diarrhea or had diarrhea with a negativity for toxin A/B. When the same patients were admitted several times due to postoperative complications or chemotherapy, they were considered new cases because their statuses were different from previous admissions.
The independent t-test was used for continuous variables and the chi-square test was used for categorical variables in order to identify statistically significant associations between the non-CDC and CDC groups. The Fisher exact test was used to analyze the results of the smaller sample sizes. Logistic regression analysis was used to determine the net effects of each independent variable while controlling for other variables. A 95% confidence interval (CI) was used to quantify the relationship of risk factors. A P-value of <0.05 was considered statistically significant.
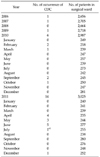
The numbers of occurrences of CDC in the surgical ward were 1 in 2006, 1 in 2007, 0 in 2008, 1 in 2009, 6 in 2010, and 14 in 2011. The rate of CDC occurrence suddenly increased between January 2010 and July 2011 (Table 1, Fig. 1). A total of 4,720 patients were admitted to the surgical ward during this period. The non-CDC group consisted of 4,701 (99.6%) and the CDC group consisted of 19 (0.4%) patients. All 19 patients in the CDC group had been managed by antibiotics before the onset of CDC. None of the patients who did not receive antibiotics were infected by C. difficile (n = 1,548, 32.8%, P < 0.001). The mean age of the patients was older in the CDC group than in the non-CDC group (66.7 ± 13.6 vs. 47.3 ± 19.2, P < 0.001). The mean duration of hospitalization was longer in the non-CDC group than in the CDC group (9.4 ± 9.6 vs. 20.6 ± 9.7 days, P < 0.001). There was no significant difference in duration of antibiotic use between the 2 groups. The duration of antibiotic use were 2.8 ± 2.5 days in the non-CDC group and 3.0 ± 2.1 days in the CDC group (P = 0.710). The therapeutic use of metronidazole or oral vancomycin for CDC was not included in the duration of antibiotic use. Of all patients, 1,977 (41.9%) were managed conservatively without surgery and 2,743 patients (58.1%) were managed by surgery. The diseases of the patients managed conservatively at the surgical ward were postoperative complications (adhesive ileus or surgical site infection), enteritis and diseases associated with the medical division. Of the 19 patients in the CDC group, 18 (94.7%) underwent surgeries. Only 1 patient of the conservatively managed patients showed CDC (P < 0.001). This patient was admitted due to ileus and improved without any surgery. After the ileus improved, the patient had diarrhea and was diagnosed with confirmed CDC (Table 2).
Eighteen patients in the CDC group were scheduled for operations. None of them had emergency operations. They all received prophylactic antibiotics for 2 to 3 days during the perioperative period. Of these patients, 2 received additional antibiotics for 7 and 10 days, respectively, due to surgical site infection, and 1 received additional antibiotics for 5 days due to urinary tract infection. In cases of scheduled colectomy, we performed bowel preparation with 4 L of polyethylene glycol and oral metronidazole before operation.
We also analyzed relationships between operation types and CDC in patients with operation. Although mastectomy and thyroidectomy were most frequently performed, CDC was most frequently occurred in patients with colectomies (P < 0.001) (Table 3). Among these patients, the incidence of CDC was 4.3% (Table 2). All patients were also classified into 2 distinct categories: the colectomy group and non-colectomy groups. The incidence of CDC was significantly higher in the colectomy group than in the non-colecotmy group (P < 0.001).
The patients undergoing chemotherapy sometimes showed leukopenia and had absolute neutrophil counts below 500. The 170 patients (3.6%) with leukopenia wore masks and consumed sterile foods. None of them had CDC.
During the survey period of 19 months, 0.9% (531/55,889) of patients showed CDC in Hallym University Sacred Heart Hospital, including the medical ward. Of these 531 patients, 197 (37.1%) had CDC in the medical ward comprising 3 floors, and 19 (3.6%) had CDC in the surgical ward comprising only 1 floor. The incidence of CDC was lower at the surgical ward than at the medical ward. The patients who were transferred from the medical ward after surgery showed a higher incidence of CDC than the other patients (6/481 [1.24%] vs. 13/4329 [0.3%], P = 0.009). There was no significant difference in the gender ratio of between these 2 categories.
We converted continuous variables, such as age and hospital days, to categorical ones for multivariate analysis. Age was differentiated at 55 years, the median value of the 2 groups. We performed multivariate analysis with significant variables obtained from univariate analysis. Multivariate analysis showed that colectomy and hospital stays longer than 10 days were high risk factors for CDC occurrence in the surgical ward. Age greater than 55 years and transference from the medical ward were not important factors for CDC in the surgical ward (Table 4).
The incidence of CDC has increased [3-6]. In the last 19 months, we experienced an abrupt increase in the occurrence of CDC in the surgical ward. The reason may be that this increase on surgical ward would be attributable to environmental contamination of the surgical ward with C. difficile spores. C. difficile spores can be transferred though items in common use. The surgical ward of our hospital was composed of 1 floor, 1 nursing station and the main aisle in the center. All patients were exposed to the same aisle, 2 public toilets, the same dressing carts and the same meal delivery carts. After recognizing increase in occurrence, we sterilized the surgical ward with disinfectants containing chlorine and emphasized the importance of washing hands with soap and water. After that, we did not experience further CDC cases for 4 months. It is well known that washing hands is more effective for the prevention of CDC than alcohol disinfectants because spores can tolerate alcohol [7]. It is important to prevent CDC through environmental decontamination and to minimize opportunities for cross-infection through hand hygiene and barrier precautions [8,9].
It has been shown that gastrointestinal surgery is a significant risk factor for the development of CDC [10-12]. Our result also showed that most patients with CDC had undergone gastrointestinal surgeries and most were colectomies (16 gastrointestinal surgeries, 1 hepatobiliary surgery and 1 chest surgery). Taslim [11] has demonstrated that preoperative bowel preparation, oral preoperative antibiotics, prolonged postoperative systemic antibiotic therapy and impaired bowel motility secondary to ileus increase the infection rate of C. difficile. Wren et al. [13] have suggested that preoperative oral antibiotics in colorectal surgery increase the incidence of CDC. In our study, since most cases of CDC occurred after colectomy, we believe that environmental changes within the colon may be important in the occurrence of CDC in patients with gastrointestinal surgeries. We performed bowel cleansing with 4 L of polyethylene glycol before the operation day as well as oral metronidazole for 2 days before the operation to prepare colecotmy. During the colectomy, we also irrigated the lumen of the bowel at an approximate length of 5 cm with a diluted betadine solution through both cut lumens or the anus. Povidone-iodine irrigation can prevent surgical site infection [14]. Such procedures for decreasing colonic bacteria and stool may change the normal flora, and allow C. difficile to settle and colonize in the gut, subsequently causing CDC.
Krapohl et al. [15] have reported that mechanical bowel preparation is not a risk factor for C. difficile infection after colecotmy. In the same study, they also stated that the use of preoperative oral antibiotics is not statistically significant in C. difficile infection. Morotomi et al. [16] have documented that oral intake of a polyethylene glycol-electrolyte lavage solution does not affect the intestinal microflora. They also described that preoperative bowel preparation with the lavage solution might not alter the normal flora of the colon. Our results showed that the colectotmy was an important high risk factor for CDC. Considering our abrupt increase in the rate of CDC during a short period, it is conceivable that all inpatients on the surgical ward may have been exposed to C. difficile spores due to environmental contamination. Although previous studies have reported a generally increased incidence, they have not shown such an abrupt increase within such a short period. In addition, the environmental condition might be different among institutions. Intra-operative procedures, such as bowel irrigation, have not been mentioned in previous reports. These differences might also affect C. difficile infection and its incidence.
The postoperative recovery time of the motility of the colon is about 3 to 5 days and that of the small bowel is within 24 hours [17]. Paralytic ileus is associated with alterations in the intestinal flora and overgrowth of bacteria [18]. During postoperative ileus, C. difficile can colonize in the colon unless the colon is washed out. It is believed that upper gastrointestinal or hepatobiliary operations with a rapid recovery time of bowel motility show lower incidences of CDC than colectomies.
Many previous studies have suggested that old age is an independent risk factor for CDC. Karlstrom et al. [19] and Yeom et al. [20] have reported that ages ≥60 years showed a high incidence of CDC. Lee et al. [21] have reported that ages ≥70 years are an independent risk factor in multivariate analysis. They also explained that the increased incidence is related to a decrease in immunity through the aging process and an increase in the incidence of underlying disease. Our study showed that ages ≥55 years were statistically not significant in multivariate analysis. Unlike previous studies, colectomy was included in our analysis. Further studies are warranted to confirm this issue.
Long hospitalizations have been reported to be an independent risk factor for CDC in many studies [21-23]. Postoperative complications, such as ileus, deep surgical site infection, leakage or urinary tract infection, can prolong hospitalization. The additional antibiotics administered due to infection may increase the incidence of CDC [11]. However, Metzger et al. [24] have shown that additional antibiotics do not increase CDC. It is thought that prolonged hospitalization may increase the chances of exposure to C. difficile spores and the incidence of CDC.
The robust antitoxin immune response counteracts toxins A and B released by pathogenic strains [25]. Immnunocompromised patients undergoing posttransplantation medication, chemotherapy or human immunodeficiency virus infection show more than 30% increase in the incidence of CDC [26]. In contrast, our study showed that none of the patients with leukopenia had CDC. Our patients with leukopenia were protected from exposure to C. difficile spores because they were asked to wash out their hands and wear a mask. They ate only sterile foods and were prohibited from eating food several from the outside. Isolation strategies can protect immunocompormised patients from CDC.
We considered transference from a medical ward as a risk factor because the incidence of CDC was higher than on a surgical ward. Although transference from the medical ward seemed to be a risk factor for CDC infection in univariate analysis, it was not an independent risk factor in multivariate analysis. Vesta et al. [27] have reported that the ward type is not a risk factor for C. difficile-associated diarrhea. It is believed that the high incidence of CDC in the medical ward may be due to the more frequent laboratory tests in that ward. When patients with gastrointestinal surgeries have diarrhea in the early stages of recovery, clinicians sometimes regard the symptom as part of the course of recovery and do not perform C. difficile toxin tests. Early postoperative diarrhea may be a symptom of CDC. CDC patients with mild diarrhea may not require any treatments other than the discontinuation of antibiotic therapy [2]. In our study, most patients with gastrointestinal surgeries received prophyalatic antibioitics for 2 or 3 days during the perioperative period. It is possible that patients with mild CDC could recover without any treatment after discontinuation of antibiotics. Therefore, we may not have included the patients with mild CDC in the study. If we performed tests for toxins A and B in all postoperative patients, our incidence of CDC may have increased.
In conclusion, the results of this study suggest that the risk factors for CDC in a surgical ward could be colectomy and the longer duration of hospitalization. Therefore, clinicians should consider the possibility of CDC when patients undergo colectomy, are admitted for a long time, and have postoperative diarrhea. Early detection of CDC helps manage such patients appropriately. It is very important to ensure the cleaning and disinfection of the equipment, to keep the environment clean and to emphasize hand hygiene for the prevention of CDC.
Figures and Tables
 | Fig. 1This figure shows the control chart of Clostridium difficile colitis rate per patient. SD, standard deviation. |
Table 2
Univariate analysis of risk factors for Clostridium difficle colitis (CDC) in patients admitted to surgical ward

References
1. Kulaylat MN, Dayton MT. Townsed CM, Beauchamp RD, Evers BM, Mattox KL, editors. Surgical complication. Sabiston textbook of surgery: the biological basis of modern surgical practice. 2008. 18th ed. Philadelphia: Saunders;358–359.
2. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994. 330:257–262.
3. McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006. 12:409–415.
4. Park HS, Han DS. Is Clostridium difficile infection increasing in Korea? Korean J Gastroenterol. 2010. 55:208–210.
5. Lee YJ, Choi MG, Lim CH, Jung WR, Cho HS, Sung HY, et al. Change of Clostridium difficile colitis during recent 10 years in Korea. Korean J Gastroenterol. 2010. 55:169–174.
6. Viswanathan VK, Mallozzi MJ, Vedantam G. Clostridium difficile infection: an overview of the disease and its pathogenesis, epidemiology and interventions. Gut Microbes. 2010. 1:234–242.
7. Jabbar U, Leischner J, Kasper D, Gerber R, Sambol SP, Parada JP, et al. Effectiveness of alcohol-based hand rubs for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol. 2010. 31:565–570.
8. Kelly CP, LaMont JT. Clostridium difficile: more difficult than ever. N Engl J Med. 2008. 359:1932–1940.
9. Oldfield EC 3rd. Clostridium difficile-associated diarrhea: risk factors, diagnostic methods, and treatment. Rev Gastroenterol Disord. 2004. 4:186–195.
10. Thibault A, Miller MA, Gaese C. Risk factors for the development of Clostridium difficile-associated diarrhea during a hospital outbreak. Infect Control Hosp Epidemiol. 1991. 12:345–348.
11. Taslim H. Clostridium difficile infection in the elderly. Acta Med Indones. 2009. 41:148–151.
12. Zerey M, Paton BL, Lincourt AE, Gersin KS, Kercher KW, Heniford BT. The burden of Clostridium difficile in surgical patients in the United States. Surg Infect (Larchmt). 2007. 8:557–566.
13. Wren SM, Ahmed N, Jamal A, Safadi BY. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg. 2005. 140:752–756.
14. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007. 50:473–481.
15. Krapohl GL, Phillips LR, Campbell DA Jr, Hendren S, Banerjee M, Metzger B, et al. Bowel preparation for colectomy and risk of Clostridium difficile infection. Dis Colon Rectum. 2011. 54:810–817.
16. Morotomi M, Guillem JG, Pocsidio J, LoGerfo P, Treat M, Forde KA, et al. Effect of polyethylene glycol-electrolyte lavage solution on intestinal microflora. Appl Environ Microbiol. 1989. 55:1026–1028.
17. Tavakkolizadeh A, Whang EE, Ashley SW, Zinner MJ. Brunicardi FC, Andersen D, Billiar T, Hunter J, Matthews J, Pollock RE, editors. Small intestine. Schwartz's principles of surgery. 2010. 9th ed. New York: McGraw-Hill;992.
18. Madl C, Druml W. Gastrointestinal disorders of the critically ill. Systemic consequences of ileus. Best Pract Res Clin Gastroenterol. 2003. 17:445–456.
19. Karlstrom O, Fryklund B, Tullus K, Burman LG. A prospective nationwide study of Clostridium difficile-associated diarrhea in Sweden. The Swedish C. difficile Study Group. Clin Infect Dis. 1998. 26:141–145.
20. Yeom CH, Cho MM, Baek SK, Bae OS. Risk factors for the development of Clostridium difficile-associated colitis after colorectal cancer surgery. J Korean Soc Coloproctol. 2010. 26:329–333.
21. Lee KS, Shin WG, Jang MK, Kim HS, Kim HS, Park CJ, et al. Who are susceptible to pseudomembranous colitis among patients with presumed antibiotic-associated diarrhea? Dis Colon Rectum. 2006. 49:1552–1558.
22. Park BS, Kim JH, Seo HI, Kim HS, Kim DH, Cho HJ, et al. Pseudomembranous colitis after gastrointestinal operation. J Korean Surg Soc. 2009. 77:106–112.
23. Crabtree T, Aitchison D, Meyers BF, Tymkew H, Smith JR, Guthrie TJ, et al. Clostridium difficile in cardiac surgery: risk factors and impact on postoperative outcome. Ann Thorac Surg. 2007. 83:1396–1402.
24. Metzger R, Swenson BR, Bonatti H, Hedrick TL, Hranjec T, Popovsky KA, et al. Identification of risk factors for the development of Clostridium difficile-associated diarrhea following treatment of polymicrobial surgical infections. Ann Surg. 2010. 251:722–727.
25. Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011. 60(Pt 8):1070–1079.
26. Morris AM, Jobe BA, Stoney M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002. 137:1096–1100.
27. Vesta KS, Wells PG, Gentry CA, Stipek WJ. Specific risk factors for Clostridium difficile-associated diarrhea: a prospective, multicenter, case control evaluation. Am J Infect Control. 2005. 33:469–472.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download