Abstract
Inflammatory myofibroblastic tumor (IMT) is an uncommon mesenchymal solid tumor commonly documented in children and young adults. Here, we report a case of IMT in colon confirmed pathologically after laparoscopic anterior resection. A 35-year-old man presented with anal bleeding after defecation for 2 weeks. Colonoscopy demonstrated a mass with shallow ulceration in the central area and irregular margin accompanied by intact mucosa in the descending colon. Computer tomography showed a well-demarcated and homogenous solitary mass in the descending colon. We performed laparoscopic anterior resection. This case was diagnosed as IMT after microscopic examination. The tumor was composed of a proliferation of spindle-shaped cells arranged in the hyaline material with chronic inflammatory cells, composed mainly of plasma cells and lymphocytes. Immunohistochemically, tumor cells were positive for smooth muscle actin, and vimentin, and negative for desmin, CD117 (c-kit), anaplastic lymphoma kinase-1.
Inflammatory myofibroblastic tumor (IMT) is an uncommon mesenchymal solid tumor commonly documented in children and young adults [1,2]. Although it occurs primarily in the lung, IMTs in many organs including stomach, small intestine, large intestine, liver, mediastinum, retroperitoneum and bladder have also been documented [1,2]. The clinical presentation is determined by the site of origin and the effects of the mass. Thus, IMT presents with non-specific clinical symptoms and its diagnosis can be difficult. IMT derived from the gastrointestinal (GI) tract presents with clinical symptoms of anemia, GI obstruction, fecal occult blood positive or intussusception. Those are not specific symptoms in IMT because other GI tract tumor may also present with similar symptoms [1,3,4]. IMT has been synonymously referred to as inflammatory pseudotumor, pseudosarcomatous myofibroblastic lesion, pseudosarcomatous fibromyxoid lesion or plasma cell granuloma. These words are synonymous and have been in common use, all sharing a key pathologic differentiation; a dominant spindle cell proliferation with a variable inflammatory component. These spindle cells are in fact myofibroblasts, thus, the preferred term for reference should be IMT reflective of the pathologic characters despite the persistence of others [5].
We report an uncommon case of 35-year-old man presenting with complaints of anal bleeding and histopathologically diagnosed with descending colonic IMT after operation.
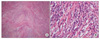
A 35-year-old man presented with anal bleeding after defecation for 2 weeks. It is not associated with the symptoms of abdominal pain, fever or weight loss. He had taken oil mixed with a trace of benzopyrene for 3 years because of his job of checking oil type in the edible oil plant. He had been treated for variant angina and underwent herniorrhaphy for inguinal hernia 12 years ago. Vital signs, physical examination, and peripheral blood analysis (hemoglobin 14.5 g/dL, hematocrit 43.2%) were in the normal range. Colonoscopy demonstrated a 4.0 cm sized-mass with shallow ulceration in the central area and irregular margin accompanied by intact mucosa (Fig. 1). Microscopic examination of biopsy specimens showed chronic inflammation. Computer tomography showed a well-demarcated and homogenous solitary mass in the descending colon. On contrast enhanced view, the mass had enhanced homogeneously in delayed phase (Fig. 2A, B). Colonoscopy was performed for clipping and tattooing and the patient underwent a laparoscopic anterior resection. On laparoscopy, a considerable amount of ascites (about 30 mL) and enlarged mesocolic lymph nodes were shown. The lymph nodes were resected and frozen biopsy concluded all lymph nodes were negative for malignancy. Because the main tumor was found to arise from descending colon, left colic branch of inferior mesenteric artery (IMA) and inferior mesenteric vein were ligated while IMA was preserved, then laparoscopic anterior resection was performed. After the main mass was resected, hand-sewing colocolic anastomosis was performed. The 3.9 × 3.8 cm sized mass grossly presented a fungating surface, white to yellow color and invaded muscularis propria (Fig. 3). Consistency of the mass was harder than adenocarcinoma. The histopathologic examination of the specimens showed the tumor was composed of a proliferation of spindle-shaped cells arranged in hyaline material with chronic inflammatory cells, composed mainly of plasma cells and lymphocytes, not neutrophils. The specimens did not have celluar atypia or hyperchromatism in the cells (Fig. 4A, B). Immunohistochemical staining suggested that fibroblastic tissue was of myofibroblastic nature. Immunohistochemically, tumor cells were positive for smooth muscle actin, and vimentin, and negative for desmin, CD117 (c-kit), anaplastic lymphoma kinase (ALK)-1 (Fig. 5A, B).
These findings were compatible with an IMT. The patient's postoperative course was uneventful, and he was discharged on the 7th postoperative day. He has been free of complication after 1 month of follow-up.
IMT was originally described in the lungs in 1937, and since then has been reported at various sites such as mesentery, stomach, small intestine, large intestine, liver, mediastinum, retroperitoneum and bladder [1,2,6]. The most common site of IMTs is the lung and the most common sites of extrapulmonary inflammatory myofibroblastic tumor are the mesentery and omentum [1,2,6,7]. Among extrapulmonary IMT, 43% arose in the mesentery and omentum [7]. Although it occurs primarily in children and young adults (mean age, approximately 10 years), in more recent years a broad age range has been documented [1]. There is no difference in incidence between females and males, though each report has a difference in mean age and gender ratio [2,5].
The etiology of IMT is still unknown. But some people think that development of IMT occurs after trauma, surgery or infection such as Epstein-Barr virus and human herpes virus related with reactive cytokine production [6,7]. A recent study reports that these lesions may possess chromosomal aberrations with resultant monoclonality and may frequently demonstrate locally aggressive behavior. As such, this entity should better be considered a neoplastic process. A chromosomal rearrangement involving 2p23, the site of the ALK gene, is present in a subset of these tumors [5]. These findings have recently shown that chromosomal abnormalities may be suggestive of clonal origin, not merely a reactive process. IMT should be considered as a true neoplasm [5].
Several cases with IMT in the colon have been confirmed in other countries. A total of 5 cases have been reported during the last ten-year period. The patients with IMT presented with anemia, intussusception, fecal occult blood positive, abdominal pain, chest pain, fever or weight loss. Except 1 case, all cases reported were in males. From 32-month-old to 79-year-old, a broad age range of IMT has been documented. The most common site of documented IMT in the colon was the right colon (ascending colon, 2 cases; cecum, 2 cases). IMT in the transverse colon (1 case) was also reported [1,3,4]. A retrospective review of the medical records was conducted at two major academic institutions over a 15-year period by Kovach et al. [5]. Review of the records from two institutions yielded 44 cases of pathologically confirmed IMT. Among these cases, only 3 cases occurred in colon [5].
Differential diagnosis of IMT includes malignancy and submucosal tumor from endoscopic findings. In this case, on endoscopic examination, we need to distinguish IMT from lymphoma and submucosal tumor composed of intact mucosal margin. Radiologically differential diagnosis of abdominal IMT includes solitary fibrous tumor and fibromatoses (desmoid tumor).
Inflammatory fibroid polyp, fibromatoses (desmoid tumor), gastrointestinal stromal tumors (GIST), leiomyoma, leiomyosarcoma, and schwannoma have similar pathological findings with IMT. Inflammatory fibroid polyp is typically submucosal and consists of a mixture of small granulation tissue-like vessels, spindle cells, and inflammatory cells (particulary eosinophils). Fibromatoses are composed of bland spindled or stellate cells, arranged in parallel with evenly spaced blood vessels and a collagenous background. Fibromatoses often have mitotic activity, but cytologic pleomorphism is generally not seen. GIST stains for CD34 and CD117 (c-kit). Leiomyoma and leiomyosarcoma stain positively for desmin and actin and negatively for CD117 and CD34. Schwannoma stains strongly for S-100 (nuclear and cytoplasmic) and is CD117 negative [1,6,8]. Immunohistochemically, strong diffuse cytoplasmic reactivity for vimentin is typical for virtually all IMT. Reactivity for smooth muscle actin and muscle specific actin varies from a focal to a diffuse pattern in the spindle cell cytoplasm, and desmin is identified in many cases [7]. Immunohistochemical cytoplasmic positivity for ALK using a variety of monoclonal antibodies is shown in approximately 50% of IMTs. However, ALK positivity is not specific for IMT [7]. Because IMT is rare and sometimes similar with malignancy, even after various examinations, it is important to distinguish IMT from other colonic tumors. IMT cannot be diagnosed by preoperation biopsy. Also, the tumor occasionally undergoes malignant transformation if not surgically excised, therefore surgical resection is the treatment of choice. We cannot exclude malignancy completely preoperatively, so it is hard to determine surgical resection margin. We have to understand IMT accurately and be careful to prevent over diagnosis and unnecessary operation and treatment.
Chemotherapy, radiation treatment, nonsteroidal anti-inflammatory drug (NSAID), steroid and cyclosporin-A have been used as treatment modalities, but surgical resection is considered as treatment of choice [5,6]. It rarely presents recurrence, metastasis or malignant transformation, and is classified as intermediate neoplasm in the World Health Organization histological typing, but it may recur locally or manifest systemic symptoms. It is for this reason that regular follow-up is necessary even though surgical resection was done [2,7].
Several studies have reported that ALK positive tumors have a less aggressive clinical course, although no clear relationship exists between ALK expression and prognosis in IMT [2,7,9,10]. In addition, there have been some studies that reported a correlation between p53 expression and prognosis [2,10]. The role of p53 reactivity in predicting the behavior of IMTs is uncertain [2,10]. The etiology of IMT is still unknown, neither reactive process nor neoplasm. And it is unclear about natural course of IMT, unless surgical resection is done.
Most IMTs behave in a benign manner after surgical resection, but it presents recurrence, metastasis or malignant transformation in some cases. It is unclear what kind of factor is associated with prognosis. Also, it is unclear what the best way of treatment of recurrence or metastasis is during long-term follow-up. We have to follow-up patients diagnosed IMT even if they have undergone surgical resection.
Figures and Tables
Fig. 1
Endoscopic examination reveals a 4 cm sized irregularly margined mass with intact mucosa (margin) and shallow ulcer (central) in descending colon.
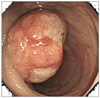
Fig. 2
Trans-axial view (A) and coronal view (B) show a 4.0 cm sized homogeneous enhancing intra-luminal mass (arrow) in descending colon.
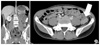
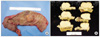
Fig. 3
Gross examination reveals a 3.9 × 3.8 cm sized, fungating, white to yellow colored and hard mass in descending colon. Mass involves muscularis propria.
References
1. Tanaka A, Hirabayashi K, Sadahiro S, Maeda Y, Suzuki T, Ogoshi K. Inflammatory myofibroblastic tumor of the ascending colon in adults manifested by positive fecal occult blood test. Gastrointest Endosc. 2010. 71:214–216.
2. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007. 31:509–520.
3. Saleem MI, Ben-Hamida MA, Barrett AM, Bunn SK, Huntley L, Wood KM, et al. Lower abdominal inflammatory myofibroblastic tumor -an unusual presentation-a case report and brief literature review. Eur J Pediatr. 2007. 166:679–683.
4. Salameh M, Sultan I, Barbar M, Al Hussaini M, Jameel A, Ghandour K, et al. Inflammatory myofibroblastic tumor causing unexplained anemia in a toddler: a case report. J Med Case Reports. 2011. 5:69.
5. Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, et al. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006. 94:385–391.
6. Karnak I, Senocak ME, Ciftci AO, Cağlar M, Bingöl-Koloğlu M, Tanyel FC, et al. Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg. 2001. 36:908–912.
7. Coffin CM, Fletcher JA. Fletcher CD, Unni KK, Mertens F, editors. Inflammatory myofibroblastic tumour. World Health Organization classification of tumours Pathology and genetics of tumours of soft tissue and bone. 2002. Lyon: IARC Press;91–93.
8. Greenson JK. Gastrointestinal stromal tumors and other mesenchymal lesions of the gut. Mod Pathol. 2003. 16:366–375.
9. Chun YS, Wang L, Nascimento AG, Moir CR, Rodeberg DA. Pediatric inflammatory myofibroblastic tumor: anaplastic lymphoma kinase (ALK) expression and prognosis. Pediatr Blood Cancer. 2005. 45:796–801.
10. Jiang YH, Cheng B, Ge MH, Cheng Y, Zhang G. Comparison of the clinical and immunohistochemical features, including anaplastic lymphoma kinase (ALK) and p53, in inflammatory myofibroblastic tumours. J Int Med Res. 2009. 37:867–877.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download