Abstract
Purpose
In contrast to proximal deep vein thrombosis (DVT), the treatment of isolated calf vein thrombosis (ICVT) remains controversial. This study aimed to investigate early treatment outcomes of ICVT after total knee arthroplasty (TKA).
Methods
Medical records of 313 patients who underwent TKA from October 2007 to December 2009 were retrospectively reviewed. A DVT-computed tomography (CT) was performed 7 days after surgery. ICVT was identified in 76 limbs of 73 patients. Of them, follow-up DVT-CT was available in 39 limbs of 37 patients. The patients with ICVTs were categorized into two groups: oral anticoagulation group (group I, 17 patients with 18 limbs) and conservative treatment group (group II, 20 patients with 21 limbs). Group I received an oral vitamin K antagonist for 3 to 6 months following low molecular weight heparin. Change of thrombus extent and development of pulmonary embolism (PE) was assessed in follow-up DVT-CT.
Results
Mean age was 68 years and 95% were female. Of 39 limbs with ICVT, 16 (41%) involved major lower leg veins (posterior tibial vein or peroneal vein), 13 (33%) involved muscular veins (soleal vein or gastrocnemius vein) and 10 (26%) involved both. During 1 to 6 months, follow-up DVT-CT revealed complete thrombus resolution in all limbs and there was no proximal propagation of thrombus or PE in both groups.
The prevalence of isolated calf vein thrombosis (ICVT) is 5 to 12% in symptomatic patients [1] and asymptomatic ICVT may occurin 15% of patients after total hip and total knee arthroplasty (TKA) [2]. However, treatment of ICVT is still controversial.
Generally, ICVT is not considered to be a source of fatal pulmonary embolism (PE) because the severity of PE depends on the size of the emboli or cardiopulmonary function [3]. However, some authors who recommend anticoagulation therapy are concerned that untreated ICVT may extend to proximal deep veins, which is more likely to result in PE [4].
This study aimed to investigate short-term treatment outcomes (e.g., proximal thrombus propagation and incidence of PE) in patients with ICVT after TKA according to whether or not they were treated with anticoagulation therapies.
Between October 2007 and December 2009, 313 TKAs were performed in Kyungpook National University Hospital. During the perioperative period, prophylactic anticoagulation was not administered and only a compression stocking was applied. On postoperative day 7, a deep vein thrombosis-computed tomography (DVT-CT) designed in our hospital was performed on all patients for venous thromboembolism (VTE) screening. The prevalence of VTE was 34% (105/313). PE without DVT was 4% (11/313) and DVT with or without PE was 30% (94/313). DVT-CT revealed ICVT in 76 limbs of 73 patients. Of them, 39 limbs of 37 patients who underwent follow-up DVT-CT were enrolled in this study. By retrospective review of prospectively registered medical records, we investigated the incidence of resolution and proximal propagation of ICVT as well as the incidence of PE.
Regarding treatment, indications of 3 to 6 months oral anticoagulation therapy were as follows: 1) concomitant PE regardless of symptom, 2) concomitant proximal DVT in contralateral limb, 3) previous episode of DVT.
The patients with ICVTs were categorized into two groups according to treatment. Seventeen patients with 18 limbs (group I, oral anticoagulation group) were treated 3 to 6 months with oral anticoagulation therapy following low molecular weight heparin (LMWH) and 20 patients with 21 limbs (group II, conservative treatment group) were not. Among group II, however, 11 patients received short-term (3 to 7 days) LMWH during admission and 15 patients received antiplatelet therapy for 3 to 6 months. Neither anticoagulation nor antiplatelet agent was administered for 4 patients.
Sixteen patients had concomitant PE and all were asymptomatic. All belonged to group I, except 1 patient who discontinued anticoagulation due to hematemesis one day after anticoagulation therapy.
CT was performed using a Light Speed 16 CT scanner (GE Healthcare, Milwaukee, WI, USA) or Aquilion 64 CT scanner (Toshiba Medical Systems Co., Tokyo, Japan). The contrast media (Optiray 320, Tyco Healthcare, Mallinkrodt, Saint Louis, MO, USA) with a volume of 140 mL was administered through an antecubital vein using an automated power injector at a rate of 3 to 4 mL/sec and was followed a 30 mL saline chaser with the same infusion rate. Individual contrast optimization was achieved using bolus tracking within the main pulmonary artery. CT pulmonary arteriography was obtained during a shallow inspiratory breathhold in a craniocaudal direction from the aortic arch to the heart base. At 180 seconds after injection of the contrast agent, CT venography was performed from the heart base down to the feet in the craniocaudal direction.
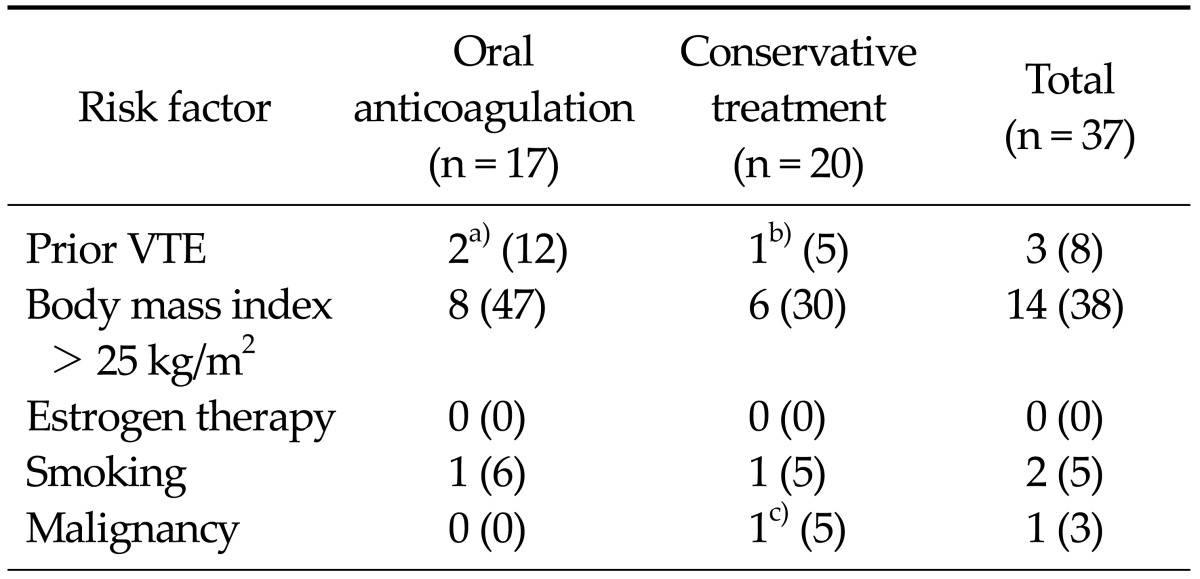
Patient mean age was 68 years (range, 48 to 86 years) and 95% were female. Two patients had bilateral ICVT and 1 patient had a contralateral proximal DVT. Sixteen patients had a concomitant PE and all were asymptomatic. The risk factors for VTE are shown in Table 1. Two patients had a previous episode of ICVT of the contralateral limb after contralateral TKA and 1 patient had undergone catheter-directed thrombolysis and left iliac vein stenting due to May-Thurner syndrome 5 years ago.
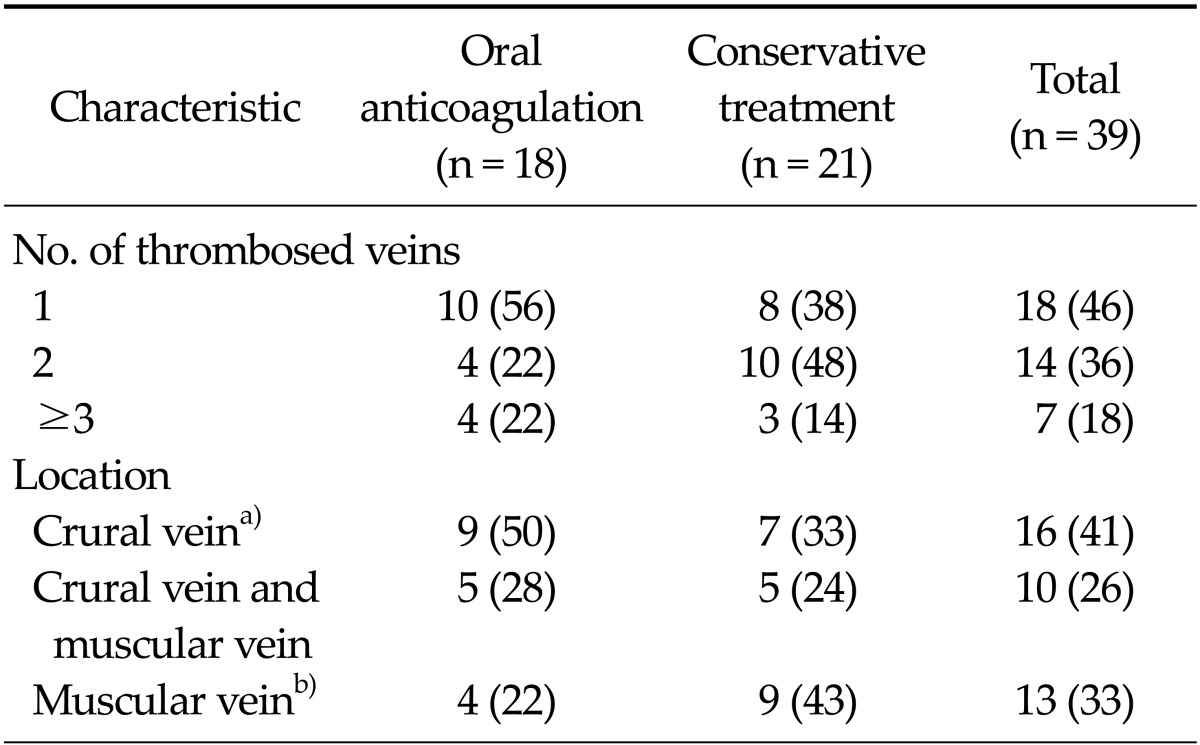
Table 2 summarizes the locations of ICVT and the number of thrombosed veins. Fifty-four percent involved more than 1 vein. Among the crural veins, peroneal vein thrombosis occurred in 16 limbs as well as the posterior tibial vein, but the anterior tibial vein was not involved in any case. Isolated muscular calf vein thrombosis (IMCVT) was seen in 33% (13/39) of patients. Follow-up DVT-CT revealed complete resolution of ICVT and PE in the oral anticoagulation group (Fig. 1). In the conservative treatment group, ICVT also disappeared on follow-up DVT-CT and there was no newly developed PE (Fig. 2).
There were many reports on ICVT, but its clinical implication and treatment remains controversial. Traditionally, main concerns in DVT treatment are the risk of PE and post-thrombotic syndrome.
There were autopsy studies demonstrating that ICVT
was a source of fatal PE [5,6]. Sevitt and Gallagher [5] performed autopsies in 74 fatal cases of PE and the rate of fatal PE from ICVT was 15% (11/74). In another autopsy study of 23 patients who died of PE, ICVT was detected in 3 patients (13%) [6]. Although the reported incidence of PE in patients with ICVT range from 7 to 50% [3,7-9], fatal PE caused by ICVT is rarely reported in clinical studies. However, 20 to 50% of untreated proximal DVT results in significant PE and 5% of such PE are fatal [10]. Therefore, another issue of ICVT is its propagation to the level of or above the popliteal vein.
Based on serial duplex scans of 192 patients with ICVT, Lohr et al. [11] reported that 28% had propagation of their initial thrombi. Propagation up to the proximal veins occurred in 21 patients (11%). They failed to identify risk factors of thrombus propagation. In a randomized prospective studyin 51 patients with ICVT diagnosed by contrast venography [12], 29% (8/28) of the non-warfarin group had recurrences and 18% (5/28) were recurrences with proximal extension during the first 3 months. However, there were no recurrences in the warfarin group (P < 0.01). After 1 year, there recurrence rate was 68% in the non-warfarin group, while there were no recurrences in the warfarin group, except for 1 patient who died of lung cancer after 4 months (P < 0.02). Lautz et al. [13] reported a mean 7.5 months follow-up result of 452 limbs in 406 patients with IMCVT. VTE event occurred in 18.7% of the patients. According to the treatment, the incidence of VTE events was significantly lower after therapeutic anticoagulation (12% in the anticoagulation group vs. 27% in the prophylactic anticoagulation group vs. 30% in the no anticoagulation group, P = 0.0003).
On the contrary, there are some reports that postoperative ICVT is insignificant. Wang et al. [14] performed ascending venography after TKA and identified 51 patients with ICVT. Symptomatic ICVT was treated with heparin or LMWH for 3 to 7 days and asymptomatic patients received no medication. Follow-up venography was performed in 37 patients. It showed no thrombosis except in 1 patient who had residual thrombi in the muscular branches. In other study [15], a serial follow-up duplex scan was performed for 42 patients (50 limbs) with ICVT after total hip arthroplasty or TKA. Its proximal propagation rate was not influenced by anticoagulation (23% in treated limbs vs. 8% in untreated limbs, P = 0.43). The authors suggested that postoperative calf vein thrombosis need not be routinely treated and serial venous duplex scanning is useful to identify thrombotic propagation requiring anticoagulation.
Masuda et al. [16] reported that proximal propagation of ICVT developed in 4% of the cases within 2 weeks of diagnosis and clinically overt PE did not occur regardless of whether anticoagulation therapy was received or not. Meissner et al. [17] monitored 29 limbs with ICVT with duplex scan. Median propagation time of ICVT was 11 days. MacDonald et al. [18] performed serial duplex scansfor 135 limbs with IMCVT that were not treated with anticoagulation therapy. Only 3% of the patients extended their thrombi to the level of the popliteal vein and all occurred within 2 weeks.
Considering most thrombus propagations occur in the early period, it is reasonable that short-term anticoagulation may be adopted for patients with ICVT. There was a report that short-term anticoagulation in IMCVT is useful to prevent VTE complications [19], but it was not proven in a prospective randomized study [20]. The authors compared treatment outcomes between a short-term anticoagulation group (treated with LMWH for 10 days) and a compression group. There was no difference in the thrombus propagation rate (3.7% in LMWH group vs. 3.8% in compression group) and there was no clinical PE and no death.
Another issue in ICVT is post-thrombotic syndrome. In several studies, long-term symptoms and significant hemodynamic changes occur after ICVT [21-23]. Browse et al. [21] reported a 20% incidence of moderate to severe postphlebitic symptoms and signs 5 to 10 years after symptomatic ICVT. Meissner et al. [22] reported that 23% of patients with ICVT showed persistent symptoms and the development of valvular incompetence at 1 year, although recanalization proceeded rapidly. In a population-based study [23], the cumulative incidence of post-thrombotic syndrome after ICVT was 10.2%, 22%, and 29.8% at 2 years, 10 years, and 20 years respectively.
We preferred 3-month oral anticoagulation therapy for patients with ICVT if a patient had a concomitant PE at initial diagnosis. On follow-up DVT-CT 3 to 6 months after the first diagnosis of ICVT, thrombus propagation was not detected and all ICVTs resolved regardless of treatment. And, there was no newly developed PE in the conservative treatment group. This result suggests that 3-month oral anticoagulation therapy for ICVT patients without PE after TKA may not be necessary concerning the risk of proximal thrombus propagation and development of PE. It is assumed that most postoperative ICVT resolve spontaneously when the postoperative period passes and the patients can ambulate because TKA is a transient risk factor for VTE.
The limitation of this study is that it is a retrospective design with no randomization. Further, long-term follow-up is necessary to evaluate disease recurrence and post-thrombotic syndrome. Indeed, to establish a consensus guideline for ICVT treatment, a large-scale prospective randomized study is necessary.
References
1. Gottlieb RH, Widjaja J. Clinical outcomes of untreated symptomatic patients with negative findings on sonography of the thigh for deep vein thrombosis: our experience and a review of the literature. AJR Am J Roentgenol. 1999; 172:1601–1604. PMID: 10350297.

2. Oishi CS, Grady-Benson JC, Otis SM, Colwell CW Jr, Walker RH. The clinical course of distal deep venous thrombosis after total hip and total knee arthroplasty, as determined with duplex ultrasonography. J Bone Joint Surg Am. 1994; 76:1658–1663. PMID: 7962026.

3. Ohgi S, Tachibana M, Ikebuchi M, Kanaoka Y, Maeda T, Mori T. Pulmonary embolism in patients with isolated soleal vein thrombosis. Angiology. 1998; 49:759–764. PMID: 9756428.

4. Lohr JM, Kerr TM, Lutter KS, Cranley RD, Spirtoff K, Cranley JJ. Lower extremity calf thrombosis: to treat or not to treat? J Vasc Surg. 1991; 14:618–623. PMID: 1942369.

5. Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism: a clinico-pathological study in injured and burned patients. Br J Surg. 1961; 48:475–489. PMID: 13750445.

6. Giachino A. Relationship between deep-vein thrombosis in the calf and fatal pulmonary embolism. Can J Surg. 1988; 31:129–130. PMID: 3349377.
7. Guias B, Simoni G, Oger E, Lemire A, Leroyer C, Mottier D, et al. Calf muscle venous thrombosis and pulmonary embolism. J Mal Vasc. 1999; 24:132–134. PMID: 10399646.
8. Hollerweger A, Macheiner P, Rettenbacher T, Gritzmann N. Sonographic diagnosis of thrombosis of the calf muscle veins and the risk of pulmonary embolism. Ultraschall Med. 2000; 21:66–72. PMID: 10838706.
9. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep's clothing? Arch Intern Med. 1988; 148:2131–2138. PMID: 3052345.

10. Deitcher SR, Caprini JA. Calf deep venous thrombosis should be treated with anticoagulation. Med Clin North Am. 2003; 87:1157–1164. PMID: 14680297.

11. Lohr JM, James KV, Deshmukh RM, Hasselfeld KA, Allastair B. Karmody Award. Calf vein thrombi are not a benign finding. Am J Surg. 1995; 170:86–90. PMID: 7631940.
12. Lagerstedt CI, Olsson CG, Fagher BO, Oqvist BW, Albrechtsson U. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet. 1985; 2:515–518. PMID: 2863541.

13. Lautz TB, Abbas F, Walsh SJ, Chow C, Amaranto DJ, Wang E, et al. Isolated gastrocnemius and soleal vein thrombosis: should these patients receive therapeutic anticoagulation? Ann Surg. 2010; 251:735–742. PMID: 19858700.
14. Wang CJ, Wang JW, Weng LH, Hsu CC, Lo CF. Outcome of calf deep-vein thrombosis after total knee arthroplasty. J Bone Joint Surg Br. 2003; 85:841–844. PMID: 12931802.

15. Solis MM, Ranval TJ, Nix ML, Eidt JF, Nelson CL, Ferris EJ, et al. Is anticoagulation indicated for asymptomatic postoperative calf vein thrombosis? J Vasc Surg. 1992; 16:414–418. PMID: 1522645.

16. Masuda EM, Kessler DM, Kistner RL, Eklof B, Sato DT. The natural history of calf vein thrombosis: lysis of thrombi and development of reflux. J Vasc Surg. 1998; 28:67–73. PMID: 9685132.

17. Meissner MH, Caps MT, Bergelin RO, Manzo RA, Strandness DE Jr. Propagation, rethrombosis and new thrombus formation after acute deep venous thrombosis. J Vasc Surg. 1995; 22:558–567. PMID: 7494356.

18. MacDonald PS, Kahn SR, Miller N, Obrand D. Short-term natural history of isolated gastrocnemius and soleal vein thrombosis. J Vasc Surg. 2003; 37:523–527. PMID: 12618686.

19. Schwarz T, Schmidt B, Beyer J, Schellong SM. Therapy of isolated calf muscle vein thrombosis with low-molecular-weight heparin. Blood Coagul Fibrinolysis. 2001; 12:597–599. PMID: 11685050.

20. Schwarz T, Buschmann L, Beyer J, Halbritter K, Rastan A, Schellong S. Therapy of isolated calf muscle vein thrombosis: a randomized, controlled study. J Vasc Surg. 2010; 52:1246–1250. PMID: 20630682.

21. Browse NL, Clemenson G, Thomas ML. Is the postphlebitic leg always postphlebitic? Relation between phlebographic appearances of deep-vein thrombosis and late sequelae. Br Med J. 1980; 281:1167–1170. PMID: 7427621.

22. Meissner MH, Caps MT, Bergelin RO, Manzo RA, Strandness DE Jr. Early outcome after isolated calf vein thrombosis. J Vasc Surg. 1997; 26:749–756. PMID: 9372811.

23. Mohr DN, Silverstein MD, Heit JA, Petterson TM, O'Fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc. 2000; 75:1249–1256. PMID: 11126832.

Fig. 1
Computed tomography (CT) of 66-year-old woman who underwent right total knee arthroplasty (TKA). She was treated with oral anticoagulation therapy for 3 months due to isolated calf vein thrombosis (ICVT) with pulmonary embolism (PE). (A) CT at 7 days after TKA revealed PE in right posterior basal segmental artery (arrow) and thrombosis in right muscular calf veins (arrow heads). (B) Follow-up CT after 4 months revealed complete resolution of ICVT and PE.
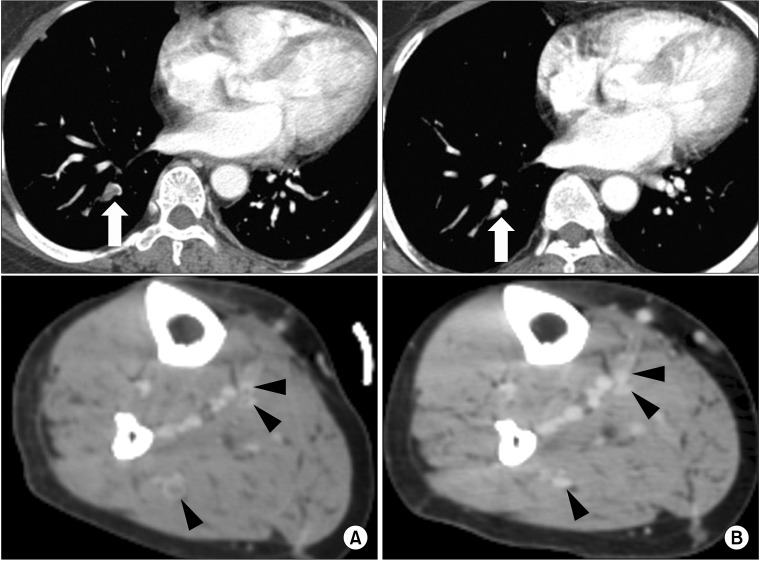
Fig. 2
Computed tomography (CT) of 67-year-old man who underwent right total knee arthroplasty (TKA). He was treated with low molecular weight heparin for 5 days during admission and took antiplatelet agent for 3 months. (A) CT at 7 days after TKA revealed thrombosis in right peroneal vein (open arrow), posterior tibial vein (solid arrow), and muscular vein (arrow head). (B) Previous thrombus disappeared on follow-up CT after 3 months.
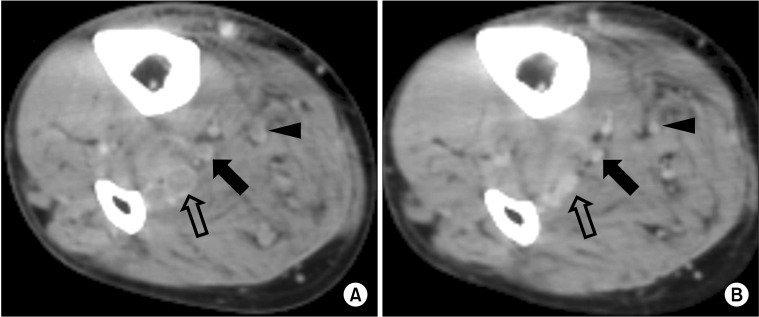
Table 1
Risk factors for venous thromboembolism (VTE) in 37 patients with isolated calf vein thrombosis (ICVT)
Values are presented as no. of patients (%).
a)One patient with May-Thurner syndrome previously underwent catheter-directed thrombolytic therapy with left iliac vein stenting and the other had ICVT after previous contralateral total knee arthroplasty (TKA). b)ICVT after previous contralateral TKA. c)Thyroid cancer.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download