Abstract
A 47-year-old man was referred to Seoul National University Bundang Hospital with an ulcerative lesion in the midbody of the stomach. Computed tomography revealed that he was a situs inversus totalis (SIT) patient. Robot-assisted distal gastrectomy with D1+β lymph node dissection and Billroth II anastomosis were performed. With the aid of robotic surgery, the surgeon didn't need to change his position and could perform the surgery without any confusion resulting from the patient's reversed anatomy. The operation took 300 minutes, with no intraoperative complications. The postoperative course was uneventful, and the patient was discharged on postoperative day 8. The final pathologic report was pT3N3a by American Joint Committee on Cancer 7th tumor-node-metastasis staging. We successfully performed robot-assisted distal gastrectomy for gastric cancer in a SIT patient. We believe that this is the first case of robotic surgery reported in a SIT patient with gastric cancer.
Situs inversus totalis (SIT) is a rare autosomal recessive congenital anomaly, occurring at an incidence of one in every 4,000 to 8,000 people. It is characterized by the transposition of the abdominal and/or thoracic organs, but it does not affect health or life expectancy. It is detected accidentally during a radiological examination. Laparoscope surgery in SIT patients with gastric cancer was first reported in 2003, and in recent years, standard typical lymph-node dissection (D1 + β) according to the gastric cancer treatment guidelines has been reported [1,2]. However, to the best of our knowledge, there has been no report of robotic surgery for gastric cancer in SIT patients. We here report a case of robot-assisted distal gastrectomy (RADG) for gastric cancer in a patient with SIT.
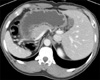
An asymptomatic ulcerative lesion was found with screening gastroscopy on the midbody of the stomach in a 47-year-old man. On endoscopic ultrasound, a 2.5 cm ulcerative lesion invading the proper muscle layer was detected in the midbody. Computed tomography (CT) revealed that the patient had SIT and an elevated lesion limited to the mucosa, without lymph-node or distant metastases (Fig. 1). Routine laboratory tests yielded normal results. The patient's preoperative body mass index was 22.5 kg/m2.
RADG with D1 + β lymph node dissection and Billroth II anastomosis were performed. Because the surgery was robotic, the surgeon did not need to change his position and could perform the operation without any confusion resulting from the patient's reversed anatomy.
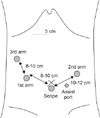
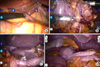
A camera was inserted into the abdominal cavity through a 12 mm port below the umbilicus, and four more trocars were inserted (Fig. 2). After a thorough examination of the internal organs (Fig. 3A), the greater omentum was divided close to the transverse colon. The right (left in the normal anatomy) gastroepiploic artery and vein were clipped and divided near the spleen. The left (right in the normal anatomy) gastroepiploic artery and vein were then clipped and divided; bifurcating from the gastroduodenal artery level (Fig. 3B). Lesser omentum was opened by harmonic, and the 1st assistant retracted liver upward. After the right (left in the normal anatomy) gastric vessels were exposed and divided near bifurcation proper hepatic artery, the duodenum was divided with a linear stapler. All vessels were clipped by the 1st assistant, not the operator. The lymph nodes around the celiac axis and the lesser curvature of the stomach were dissected (Fig. 3C). At that time, the pancreas was pulled downward by the 1st assistant. Before making the incision, we grasped the proximal jejunum about 20 cm from Treiz' ligament by locking grasper. The operator made a 5 cm mini-laparotomy in the epigastrium area. The stomach was extracted through the mini-laparotomy wound and resected at the level of the upper one-third of the stomach. A loop of the jejunum was brought up to the greater curvature side, and a routine, hand sewn Billroth II anastomosis was performed extracorporeally (Fig. 3D). The operation time was 300 minutes with no intraoperative complications. The postoperative course was uneventful, and the patient was discharged on postoperative day 8.
The final pathology showed a 4.0 cm2 × 3.5 cm2 poorly differentiated adenocarcinoma with subserosal invasion into the posterior wall of the midbody. Forty lymph nodes were examined, and 10 regional lymph nodes showed metastases (pT3N3a, IIIB according to the American Joint Committee on Cancer, 7th edition). The patient underwent adjuvant chemotherapy and has had no recurrence for 15 months.
Open surgery for gallstones or acute appendicitis in SIT patients has been reported [3,4]. Recently, laparoscopic surgery, including cholecystectomy, appendectomy, and sigmoidectomy, have been performed successfully in SIT patients [5,6].
In 1936, Allen [7] described a case of gastric cancer in a SIT patient, who died three weeks after a gastrectomy. There is no concrete evidence of a relationship between SIT and gastric cancer and there are no reports on gastric cancer incidence rates in SIT patients [8]. A successful operation for gastric cancer in a SIT patient was recently reported, and laparoscopic surgery for gastric cancer in a SIT patient has also been reported [1]. A 76-year-old man with SIT who developed early gastric cancer was successfully treated by laparoscopy-assisted distal gastrectomy (LADG). This case suggested that LADG is an alternative technique for the treatment of malignancies in patients with SIT. However, in that case report, there was no mention of lymph node dissection, which is essential during malignant surgery.
In 2010, LADG with standard lymph-node dissection (D1 + β) for early gastric cancer was successfully performed [2]. This was the first case of standard lymph node dissection in a gastric cancer patient with SIT. In Japanese gastric cancer treatment guidelines published in 2011 [9], D2 dissection (D1+ 8a, 9, 11p, 12a) is recommended in advanced gastric cancer. This case was operated on May 2010. 14v lymph node was included in the D2 dissection in that time standard, however, we did not dissect 14v. In fact, this case is sufficient to be called a 2011 D2 dissection standard. But, it was not satisfactory according to D2 dissection in 2010 standards. So we called the operation, not D2 dissection, but D1 + β. 11p and 12a lymph node dissection was done successfully.
In this case, surgery was performed by moving the monitor to the right or left while the surgeon stood on the left side, opposite the usual side for surgery. In the present case, because the surgery was robotic, the surgeon did not have to change his position and could change hands easily.
In SIT patients operation, the most important thing is to recognize anatomic variation through preoperative imaging, such as CT. Before operation, thorough examination is needed, especially vessel anatomy.
Robotic surgery is rapidly replacing conventional open or laparoscopic surgery. A large series of robot-assisted gastrectomies for gastric cancer has recently been reported [10]. The reports demonstrated that robotic surgery for gastric cancer is a safe and feasible alternative procedure in laparoscopic surgery. As exemplified by our patient, robotic surgery can be successfully used to treat gastric cancer in SIT patients.
Figures and Tables
References
1. Yamaguchi S, Orita H, Yamaoka T, Mii S, Sakata H, Hashizume M. Laparoscope-assisted distal gastrectomy for early gastric cancer in a 76-year-old man with situs inversus totalis. Surg Endosc. 2003. 17:352–353.
2. Futawatari N, Kikuchi S, Moriya H, Katada N, Sakuramoto S, Watanabe M. Laparoscopy-assisted distal gastrectomy for early gastric cancer with complete situs inversus: report of a case. Surg Today. 2010. 40:64–67.
3. Kapoor R, Dhanoa J, Afzal L, Verghese M, Jacob S. Cholecystectomy under regional anesthesia in a patient with total Kartagener's syndrome. Indian J Gastroenterol. 1997. 16:64–65.
4. Akbulut S, Caliskan A, Ekin A, Yagmur Y. Left-sided acute appendicitis with situs inversus totalis: review of 63 published cases and report of two cases. J Gastrointest Surg. 2010. 14:1422–1428.
5. Djohan RS, Rodriguez HE, Wiesman IM, Unti JA, Podbielski FJ. Laparoscopic cholecystectomy and appendectomy in situs inversus totalis. JSLS. 2000. 4:251–254.
6. Kobus C, Targarona EM, Bendahan GE, Alonso V, Balague C, Vela S, et al. Laparoscopic surgery in situs inversus: a literature review and a report of laparoscopic sigmoidectomy for diverticulitis in situs inversus. Langenbecks Arch Surg. 2004. 389:396–399.
7. Allen FR. A case of malignant tumor of the stomach in a male with transposition of the viscera. Indian Med Gaz. 1936. 71:32.
8. Yoshida Y, Saku M, Masuda Y, Maekawa S, Ikejiri K, Furuyama M. Total gastrectomy for gastric cancer associated with situs inversus totalis. A report of 2 cases. S Afr J Surg. 1992. 30:156–158.
9. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011. 14:113–123.
10. Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009. 249:927–932.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download