Abstract
Neurofibromatosis type I (NF-1) is a rare autosomal dominant genetic disorder occurring in 1 in 3,000 individuals. Vasculopathy is a rarely reported finding in patients with NF-1. Here, we report a case of recurrent aortic pseudoaneurysm after endovascular aneurysm repair in a 49-year-old male patient with NF-1. On the sixth postoperative day following a successful open surgical repair of an aortic pseudoaneurysm, he developed hemoperitoneum due to a delayed rupture of the mesenteric artery branch. This was treated with endovascular coil embolization. We report the clinical features and histologic findings of this rare vascular disorder with a review of the relevant literature.
Neurofibromatosis type I (NF-1) is an autosomal dominant genetic disorder that results from a mutation of the neurofibromin 1 gene, located on the long arm of chromosome 17 (17q11.2) [1]. NF-1 is the most common subtype of neurofibromatosis with a prevalence of approximately 1 in 3,000 births [2]. The disease has a variable phenotype, with typical characteristics including multiple café au lait spots on the skin, axillary and inguinal freckling, multiple discrete dermal neurofibromas, and iris hamartomas (Lisch nodules). Other clinical features include learning disabilities and skeletal abnormalities.
The term "NF-1 vasculopathy" has been used by medical geneticists to describe the vascular lesions seen in NF-1 [1]. The frequency of NF-1 vasculopathy is difficult to determine, however, the prevalence of vascular lesions in NF-1 patients ranges from 0.4 to 6.4% according to previously reported large clinical series [1]. Most patients with NF-1 vasculopathy are reported to be asymptomatic, despite the involvement of multiple vessels. Symptoms of vasculopathy usually occur in childhood or early adulthood. The renal artery is the most frequent site of involvement and renovascular hypertension is the most common clinical presentation. Abdominal aortic coarctation, internal carotid artery aneurysms, and cervical vertebral arterio-venous fistulae are other clinical features of NF-1 vasculopathy [1].
There have been small numbers of case reports of open surgical repair in NF-1 patients presenting with spontaneous aortic rupture [3-5]. Common surgical findings include difficulties during aortic anastomosis due to friable aortic walls and easy bleeding surrounding soft tissue. Recently, endovascular aneurysm repair (EVAR) was also reported in a NF-1 patient presenting with spontaneous aortic rupture [6].
Due to the rarity of NF-1 vasculopathy, clinical features, underlying etiologies, and optimal management have not yet been well described. Here, we report a case of NF-1 vasculopathy presenting with an infrarenal aortic pseudoaneurysm.
A 49-year-old man presented to an outpatient clinic with a saccular-shaped aneurysm at the distal infrarenal aorta. Two months prior, he had visited the emergency department of an outside hospital with a weeklong history of mid-abdominal and lower back pain with gross hematuria.
An abdominal computed tomography (CT) scan demonstrated a 3 × 3.5 cm saccular-shaped aneurysm at the infrarenal aorta 1 cm proximal to the aortic bifurcation with a 7 × 10 cm retroperitoneal hematoma surrounding the aneurysm; there was mild aneurysmal change of the left common iliac artery (Fig. 1A, B).
EVAR had been attempted at an outside hospital to treat the aortic pseudoaneurysm with contained rupture. A 20 mm diameter ×6 cm length SEAL device (S&G Biotech Inc., Seongnam, Korea) was deployed at the infrarenal aorta to exclude the rupture site. Then, a 12 mm diameter ×9 cm length SEAL stent graft (S&G Biotech Inc.) was deployed in the left iliac artery to treat the small aneurysmal dilatation of the left common iliac artery. On the final aortogram, there was no observable endoleak. The patient recovered uneventfully and was discharged from the hospital.
On a follow-up CT scan (Fig. 1C), a small pseudoaneurysm was detected distal to the aortic stent graft, prompting referral to our facility. On physical examination, multiple cutaneous nodules and café au lait spots were observed on the skin of the chest and abdomen. On abdominal X-ray there was remarkable scoliosis and lateral spondylolisthesis at the level of L3-4. His past medical history was significant for treatment with anti-tuberculosis agents while in high school for the clinical diagnosis of spinal tuberculosis, without identification of the suspected microbe.
On admission to our hospital, the patient's vital signs were stable and there was no evidence of acute retroperitoneal hemorrhage. We decided to perform an open surgical repair of the recurrent aortic aneurysm based on the relatively young age of the patient and the uncertain aortic pathology due to his underlying NF-1. We removed the aortic and iliac stent grafts and reconstructed the aorta using a Dacron graft (Boston Scientific Inc., Natick, MA, USA). Intra-operatively, easy bleeding from the retroperitoneal periaortic tissue due to increased vascularity was observed. A 2 × 3 cm pseudoaneurysm was identified at the anterolateral aspect of the terminal abdominal aorta at the level of aortic bifurcation.
On histologic examination (Fig. 2), the aortic wall close to the rupture site demonstrated medial thinning and disruption of the aortic wall, as well as degeneration of elastic fibers in the aortic wall, adventitial fibrosis, and mild inflammatory infiltrations. Focal intimal hyperplasia, adventitial hemorrhage, and foreign-body granulomas were also found in the aortic wall specimen. Although some scattered S100 protein positive spindle cells were detected in the adventitia on immunohistochemical staining, definitive histologic evidence of neurofibroma invasion of the aortic wall was not identified.
Prior to sudden onset abdominal distension and hypovolemic shock on the sixth postoperative day, the patient was hemodynamically stable following open surgical repair of the aortic aneurysm. Following his clinical deterioration, an abdominal CT scan demonstrated a large hemoperitoneum with active bleeding from a branch of the middle colic artery. The active bleeding was successfully treated with coil embolization of the middle colic artery branch proximally and distally using a microcatheter technique (Fig. 3). Intra-peritoneal blood was removed with 5 days of percutaneous drainage with a catheter at the pouch of Douglas. The patient recovered and was discharged without further complications. Cultures for bacteria, mycobacterium, and fungus from aortic wall and periaortic retroperitoneal soft tissue samples were negative.
NF-1 vasculopathy is a rare clinical feature of NF-1. Due to the silent nature of most vascular lesions and the poor accessibility of involved vessels to clinical examination, there may be an underappreciation of their occurrence. According to an autopsy series of patients with NF-1 who died of other causes, vascular abnormalities were reported in 8/18 cases (44%) [7]. Spontaneous hemothorax and retroperitoneal hematomas secondary to spontaneous aortic ruptures have been described in patients with NF-1 [3,5].
According to previous reports, underlying causes of aortic rupture in patients with NF-1 were attributed to associated connective tissue anomalies. Two distinct pathogenic mechanisms have been described. One is smooth muscle (mesodermal) dysplasia and direct vascular invasion by neurofibromatous tissue. In small vessels, there is intimal proliferation of spindle cells with secondary degenerative changes, including fibrosis, loss of media smooth muscle, and elastin fragmentation. The second suggested mechanism of aneurysm formation in patients with NF-1 is often seen in larger vessels where neurofibromatous or ganglioneuromatous tissue invades and weakens the arterial wall [8]. In the current case, the aortic wall close to the rupture site demonstrated medial thinning and disruption with degeneration of elastic fibers and adventitial fibrosis with mild inflammatory infiltration. We were unable to identify definitive histologic evidence of tumor cell invasion in the aortic wall despite finding scattered S100 protein positive spindle cells in the adventitia by immunohistochemical staining. S100 proteins are homodimeric and normally present in cells derived from the neural crest, including Schwann cells, melanocytes, glial cells, chondrocytes, adipocytes, myoepithelial cells, macrophages, Langerhans cells, dendritic cells, and keratinocytes. S100 is often used as a tumor marker and to identify epidermal differentiation [9,10]. However, S100 can also be a marker of inflammation. Therefore, positive S100 protein staining cannot be a pathognomonic finding of NF-1 infiltration in the aortic wall.
Open surgical repair for the treatment of spontaneous aortic rupture in patients with NF-1 has been reported by several authors. Shimizu et al. [3] reported a case of a 51-year-old male with neurofibromatosis presenting with a spontaneous aortic rupture, uncontrollable on emergent laparotomy, leading to his death. A report by Hines et al. [5] described a ruptured infrarenal aorta leading to hemodynamic collapse in a 52-year-old male. In this case, the aorta was found to be so friable that pledgeted sutures through the aortic wall could not be placed and the patient developed uncorrectable coagulopathy due to massive intraoperative hemorrhage, leading to early postoperative death. A successful open repair of an infrarenal aortic dissection and rupture in a 34-year-old male with neurofibromatosis was described by Chew et al. [4]. At laparotomy, there was a large tear in the infrarenal aorta just proximal to the iliac bifurcation. The defect was repaired with pledgeted sutures [4]. Considering the risk of an associated friable aortic wall, we selected normal appearing aorta for the proximal and distal anastomotic sites.
Endovascular repair of an arterial rupture in a patient with NF-1 has been reported by Falcone et al. [6]. This patient was stable following EVAR, but deteriorated and expired suddenly on the eighth postoperative day. On autopsy, it was noted that the original aortic wall defect was covered by the stent graft, however, there intimal erosion at the inferior border of the aortic endograft, similar to the current case.
We observed profuse bleeding from the soft tissue around the periaortic tissue due to increased vascularity. However, we did not identify aortic wall fragility during the aortic anastomosis, possibly due to selection of grossly healthy-appearing aortic wall for the anastomosis.
Unexpected massive intraperitoneal hemorrhage developed as a postoperative complication due to delayed rupture of a mesenteric artery branch. There was no specific injury or gross hematoma in the mesentery noted during the operation. We suspect that routine retraction of the bowel and its mesentery with a self-retaining retractor may have led to injury of the friable blood vessels and a delayed rupture of the mesenteric artery branch. To avoid this unexpected complication in the future, precautionary measures, such as gentle, atraumatic, and delicate handling of tissues, careful placement of retractors, and the use of soft, protected arterial clamps during operations in patients with NF-1 should be observed.
In conclusion, we recommend a high index of suspicion for spontaneous aortic rupture when encountering a sudden onset of abdominal or back pain with signs of blood loss in patients with NF-1. For treatment of spontaneous aortic ruptures in patients with NF-1, we recommend open surgical repair using grossly healthy-appearing aortic segments as anastomotic sites, as opposed to EVAR. During open aortic surgery, gentle and minimally traumatic handling of the vascular and soft tissues is recommended to avoid iatrogenic vessel injury.
Figures and Tables
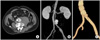 | Fig. 1(A, B) Preoperative computed tomography (CT) scan shows retroperitoneal hematoma and saccular-shaped pseudoaneurysm at left lateral wall of distal abdominal aorta at 1 cm proximal to aortic bifurcation. (C) On follow-up CT scan, small pseudoaneurysm was detected distal to aortic stent graft. |
 | Fig. 2(A) Microscopic section of aorta shows medial destruction with elastic fiber degeneration (upper left), thrombus in lumen, and adventitial fibrosis (Movat pentachrome stain, ×100). (B) S-100 protein-positive cells (arrow) are detected in adventitia of aorta (×200). |
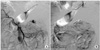 | Fig. 3(A) Mesenteric angiography on 6th day after open surgical repair of aortic aneurysm shows pseudoaneurysm formation at small branch of middle colic artery. (B) With coil embolization of proximal and distal segments of artery to pseudoaneurysm, bleeding from middle colic artery was successfully treated. |
References
1. Friedman JM, Arbiser J, Epstein JA, Gutmann DH, Huot SJ, Lin AE, et al. Cardiovascular disease in neurofibromatosis 1: report of the NF1 Cardiovascular Task Force. Genet Med. 2002. 4:105–111.
2. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, Md., USA, July 13-15, 1987. Neurofibromatosis. 1988. 1:172–178.
3. Shimizu T, Yamazaki Y, Tomoe H, Nishino S, Toma H, Shibata T, et al. Giant retroperitoneal hematoma in a patient with von Recklinghausen's disease. Nihon Hinyokika Gakkai Zasshi. 1998. 89:846–849.
4. Chew DK, Muto PM, Gordon JK, Straceski AJ, Donaldson MC. Spontaneous aortic dissection and rupture in a patient with neurofibromatosis. J Vasc Surg. 2001. 34:364–366.
5. Hines GL, Lefkowitz L, Mohtashemi M. Infrarenal aortic rupture secondary to neurofibromatosis. Ann Vasc Surg. 2002. 16:784–786.
6. Falcone JL, Go MR, Baril DT, Oakley GJ, Makaroun MS, Chaer RA. Vascular wall invasion in neurofibromatosis-induced aortic rupture. Vasc Endovascular Surg. 2010. 44:52–55.
7. Salyer WR, Salyer DC. The vascular lesions of neurofibromatosis. Angiology. 1974. 25:510–519.
8. Saitoh S, Matsuda S. Aneurysm of the major vessels in neurofibromatosis. Arch Orthop Trauma Surg. 1998. 117:110–113.
9. Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. 2008. 35:1014–1019.
10. Wolf R, Ruzicka T, Yuspa SH. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: highly homologous but distinct in regulation and function. Amino Acids. 2011. 41:789–796.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download