Abstract
Purpose
Small-for-size syndrome (SFSS) is a major problem in liver surgery, and splenectomy has been used to prevent SFSS. However, it is unknown whether splenectomy has the same effect on liver regeneration in both standard and marginal hepatectomy. The aim of this study is to see a difference in effect of splenectomy on liver regeneration according to the amount of liver resection.
Methods
Thirty male Sprague-Dawley rats (220 to 260 g) were divided into the following five groups: control (n = 6), 70% hepatectomy (n = 6), 70% hepatectomy with splenectomy (n = 6), 90% hepatectomy (n = 6), and 90% hepatectomy with splenectomy (n = 6). The animals were euthanized 24 hours after surgery and liver specimens were obtained. To assess liver regeneration, we performed immunohistochemistry of liver tissue using 5-bromo-2-deoxyuridine (BrdU) labeling and Western blot analysis of hepatic growth factor (HGF) and transforming growth factor-β (TGF-β) in the liver tissue.
Results
The splenectomized subgroup had a higher BrdU-positive cell count in the 90% hepatectomy group, but not in the 70% hepatectomy group (P < 0.001). Splenectomy significantly decreased TGF-β expression (P = 0.005) and increased the HGF to TGF-β ratio (P = 0.002) in the 90% hepatectomy group, but not in the 70% hepatectomy group.
Insufficient recovery of liver function after extended hepatectomy or partial liver transplantation, namely small-for-size syndrome (SFSS), is a major problem in liver surgery [1,2]. Because there is no effective treatment for the syndrome, prevention is critical. However, manipulation to enhance liver regeneration in the early postoperative period might serve as an attractive option when there is no way to minimize liver injury [3-6]. Among the approaches used to enhance liver regeneration, splenectomy has been used in both experimental and clinical settings, but the mechanism by which it enhances regeneration is still unclear [7-11].
It is not known whether splenectomy has an effect in both standard and marginal hepatectomy models. We hypothesized that the response after splenectomy is dependent on the extent of liver injury; splenectomy may have a beneficial effect only when there is significant liver injury. If the effect of splenectomy differs according to the amount of liver removed during hepatectomy, the clinical strategy for preemptive splenectomy in SFSS should be modified accordingly. The aim of this study is to see difference of the effect of splenectomy on liver regeneration according to the amount of liver resection, namely, in standard (70%) hepatectomy and marginal (90%) hepatectomy in rat models.
Ten-week-old male Sprague-Dawley rats (body weight, 220 to 260 g; Orient Co., Seongnam, Korea) were housed in standard animal laboratories under controlled temperature (23 ± 2℃) with a 12-hour light-dark cycle and ad libitum access to tap water and standard laboratory chow until 12 hours before the experiment when the animals were fasted. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. The experiments were approved by the Institutional Animal Care and Use Committee of Kyung Hee University.
Thirty rats were divided into three groups: the control group, the 70% hepatectomy group, and the 90% hepatectomy group. The hepatectomy groups were divided into two subgroups according to the performance of splenectomy, yielding a total of five groups: control (n = 6), 70% hepatectomy (n = 6), 70% hepatectomy with splenectomy (n = 6), 90% hepatectomy (n = 6), and 90% hepatectomy with splenectomy (n = 6).
All procedures were performed with the rats under anesthesia induced by isoflurane inhalation (isoflurane concentration, 1.5 to 3%; oxygen flow 0.5 L/min). A transverse incision was made in all animals. For the 70% hepatectomy, the interlobular ligament was dissected, and the left lateral and median lobes were resected according to the methods described by Higgins and Anderson [12]. For the 90% hepatectomy, additional resections of the right superior and right inferior lobes were performed according to procedures reported by Gaub and Iversen [13]. Splenectomy was performed using 5-0 silk ligatures for the vascular pedicles.
All animals received subcutaneous injections of the following: 5 mL of 10% glucose, 0.1 mL analgesics (Ketorolac, Whanin Pharm Co., Seoul, Korea) and 0.1 mL antibiotics (Rosephin, Roche Korea Co., Seoul, Korea). Animals had ad libitum access to 20% glucose solution for drinking and standard laboratory chow. One hour before sacrifice, 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich Co., St. Louis, MO, USA) was injected intraperitoneally (50 mg/kg).
Body weight was measured before the first operation. The remnant liver was removed and weighed immediately after sacrifice. The liver weight index was defined as the remnant liver weight divided by body weight and was expressed as a percentage.
Twenty-four hours after the surgery, the rats were deeply anesthetized using Zoletil 50 (10 mg/kg, i.p.; Virbac Co., Fort Worth, TX, USA) and blood samples were drawn from the aorta and liver tissue was obtained. Animals were euthanized by exsanguination.
MTT, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide blood samples were used for blood counts. We also measured the following serum parameters to evaluate liver function: aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, direct bilirubin, lactate dehydrogenase, and ammonia. The serum was stored at -20℃ until measurement.
Normal saline was perfused through the portal vein and the regenerated liver tissue was removed. The livers were fixed in 4% paraformaldehyde, dehydrated in graded ethanol, treated in xylene, and infiltrated and embedded in paraffin. Coronal sections (5-µm-thick) were cut using a paraffin microtome (Thermo Fisher Scientific Inc., Waltham, MA, USA), mounted on coated slides, and then dried at 37℃ overnight on a hot plate. An average of six sections were prepared for each liver.
5-bromo-2'-deoxyuridine (BrdU)-specific immunohistochemistry was performed to detect newly generated hepatocytes in the liver. Slides of paraffin-embedded liver sections were deparaffinized in xylene and rehydrated in graded alcohol solutions followed by a 5 minutes wash under running water. Tissues were denatured by boiling for 10 minutes in 10 mM citric acid (pH 6.0) and then incubating at room temperature for 10 minutes. The sections were first permeabilized by incubating in 0.5% Triton X-100 in phosphate buffered saline (PBS) for 20 minutes, then pretreated in 50% formamide-2x standard saline citrate at 65℃ for 2 hours, denatured in 2 N HCl at 37℃ for 30 minutes, and rinsed twice in 100 mM sodium borate (pH 8.5). Afterwards, the sections were incubated overnight at 4℃ with BrdU-specific mouse monoclonal antibody (1:600; Roche Korea Co.). The sections were then washed three times with PBS and incubated with biotinylated mouse secondary antibody (1:200; Vector Laboratories Inc., Burlingame, CA, USA) for 1 hour and with an avidin-peroxidase complex (1:100; Vector Laboratories Inc.) for another hour. For visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.03% DAB and 0.03% hydrogen peroxide for 5 minutes. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount. Liver regeneration index was defined as the number of BrdU-positive hepatocytes in 1 mm2.
Collected liver tissues were immediately frozen at -70℃. The tissues were homogenized with lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 10 % glycerol, 1% Triton X-100, 1.5 mM MgCl2·6H2O, 1 mM ethylene glycol tetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na2VO4, and 100 mM NaF, and then centrifuged at 14,000 rpm for 30 minutes. The protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA) and 50 µg of protein was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred onto a nitrocellulose membrane. Rabbit GAPDH antibody (1:5,000; AbFrontier, Seoul, Korea), rabbit HGF antibody (1:1,000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and mouse transforming growth factor β1 (TGF-β1) antibody (1:1,000; Serotec Ltd., Oxford, UK) were used as primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibody (1:2,000; Vector Laboratorie Inc.) was used as the secondary antibody for TGF-β1 while anti-rabbit antibody (1:3,000; Vector Laboratories Inc.) was used for GAPDH and HGF. All experiments were performed in normal lab conditions and at room temperature except for the membrane transfer. Membrane transfer was performed at 4℃ with a cold pack and pre-chilled buffer. Bands were detected using the enhanced chemiluminescence detection kit (Santa Cruz Biotechnology Inc.). To compare protein expression, the detected bands were quantitated densitometrically using Molecular Analyst version 1.4.1 (Bio-Rad Laboratories Inc.).
Data are presented as the mean ± standard error of the mean. Kruskal-Wallis analysis of variance by ranks and the Mann-Whitney test with Bonferroni correction were performed. Data were considered significant when P < 0.05. All statistical analyses were performed using SPSS ver. 13 (SPSS Inc., Chicago, IL, USA).
The differences in liver weight indexes according to the amount of resected liver were significant (P < 0.001). However, the differences between the splenectomy subgroups were not significant (Fig. 1).
The measured values for serum biochemistry are summarized in Table 1. Among the three liver resection groups, total bilirubin, direct bilirubin, AST, and ALT were significantly higher in the 70% hepatectomy group compared to the control group. Serum albumin was significantly lower in the 70% hepatectomy group compared to the control group. Meanwhile, total bilirubin, direct bilirubin, AST, ALT, and ammonia were significantly higher in the 90% hepatectomy group compared to the 70% hepatectomy group. However, the differences between the splenectomy subgroups were not significant in any group. In addition, platelet counts were not significantly different among the groups.
Liver regeneration indexes were significantly different according to the amount of liver resected (P < 0.001) (Fig. 2B). The difference between the splenectomy subgroups was significant only in the 90% hepatectomy group (P < 0.001) (Fig. 2C). Splenectomy did not significantly affect liver regeneration in the 70% hepatectomy group.
The relative expression of HGF was significantly higher in the hepatectomized groups than in the control group (P < 0.001) (Fig. 3) and also in the 90% hepatectomy group compared to the 70% hepatectomy group (P < 0.001). However, the level of HGF expression was not significantly different between the splenectomy subgroups. TGF-β expression was also significantly higher in the 70% and 90% hepatectomized groups than in the control group (P < 0.001). However, the difference was not significant between the 70% and 90% hepatectomy groups. Subgroup analysis showed that TGF-β expression was significantly lower in the splenectomy subgroup of the 90% hepatectomy group (P = 0.005), but not in the 70% hepatectomy groups. To determine the relative balance between HGF and TGF-β levels, the relative expression ratio of HGF to TGF-β was also determined. The HGF to TGF-β ratio was not significantly different between the control group and the 70% hepatectomy group, but it was significantly higher in the 90% group (P = 0.004). The subgroup analysis showed that splenectomy significantly increased the HGF to TGF-β ratio in the 90% hepatectomy group (P = 0.002), but not in 70% hepatectomy group.
It is thought that 99.95 to 99.99% of hepatocytes are in a state of growth arrest in a normal liver [14]. When there is a liver injury, it immediately induces liver regeneration, and the degree of liver regeneration is directly correlated with the amount of liver resected [15-17]. A variety of molecules are known to be involved in the control of the liver regeneration process. HGF is a well-known liver growth factor, and TGF-β is a known suppressor of liver regeneration. There is a theory that the state of liver regeneration is maintained by the balance of tonic antagonism between HGF and TGF-β [18,19]. Moreover, it has been reported that the blockade of TGF-β leads to the initiation of hepatocyte DNA synthesis in normal, unoperated rats [18]. Interestingly, the spleen is reportedly an important source of TGF-β during liver regeneration [20]. Splenectomy is known to protect the liver against ischemia/reperfusion injury and SFSS [8,21,22]. Splenectomy also has a positive effect on liver regeneration after hepatectomy and the mechanism has been explained by TGF-β reduction [20], reduction of portal venous blood flow [23], increased HO-1 and decreased TNFα [7] or sufficient oxygen supply [11]. However, previous researchers used one of the fixed type of hepatectomy among 70%, 85% or 90% models, and it is unknown whether the effect of splenectomy is the same, regardless of the extent of liver injury.
The results of this experiment showed that the effect of splenectomy on liver regeneration differs according to the extent of liver resection. The liver regeneration index measured by the number of BrdU-positive hepatocytes was significantly increased by splenectomy in the 90% hepatectomy group, but not in the 70% hepatectomy group. To rule out the possibility that liver regeneration was enhanced by increased injury caused by the splenectomy, we also assessed the extent of hepatocellular damage by analysis of aminotransferase levels after surgery. The results showed that the enzyme levels were not different according to splenectomy status, even though they were significantly different according to the amount of liver removed during hepatectomy (Table 1).
To elucidate the effect of splenectomy on liver regeneration, we studied HGF and TGF-β, two molecules with opposite actions. As mentioned before, the liver regeneration index was increased in the liver resection groups. The liver resection groups were further divided into subgroups based on whether or not they would undergo splenectomy. The relative ratio of HGF expression to TGF-β expression (HGF to TGF-β ratio) was not significantly different among the subgroups without splenectomy, indicating that the ratio was not affected by the hepatectomy itself (Fig. 3G, bars marked with an asterisk). In the 70% hepatectomy group, splenectomy did not significantly change the HGF to TGF-β ratio or the liver regeneration index. However, in the 90% hepatectomy group, splenectomy significantly increased the HGF to TGF-β ratio as well as the liver regeneration index. When hepatectomy was performed without splenectomy, both HGF and TGF-β increased, and the HGF to TGF-β ratio remained the same. However, when splenectomy was performed in conjunction with 90% hepatectomy, TGF-β expression dropped significantly while the increase in HGF expression was not significant. This drop in TGF-β expression explains the increase in the HGF to TGF-β ratio after splenectomy in 90% hepatectomy. These results demonstrate that the effect of splenectomy differs according to the amount of liver resected.
The dynamic response of liver regeneration after splenectomy has never been suggested before, and we attempted to determine the mechanism underlying this phenomenon. This phenomenon might be explained by the balance of tonic antagonism between HGF and TGF-β. Our results suggest a dynamic relationship between the amount of liver injury and the effect of splenectomy on liver regeneration. If splenectomy can be used as a preventive measure for SFSS, we may expect a greater response in patients undergoing a more extensive liver resection. Further research is needed to clarify the mechanism underlying the effects of splenectomy on SFSS.
Figures and Tables
 | Fig. 1(A) Liver weight index (the percent of remnant liver weight measured 24 hours after the first surgery divided by body weight) was significantly different according to the amount of liver resection (P < 0.001). (B) The differences between subgroups according to the splenectomy were not significant. 70 (90)% ± S, 70 (90)% hepatectomy with and without splenectomy; 70 (90)%, 70 (90)% hepatectomy without splenectomy; 70 (90)% + S, 70 (90)% hepatectomy with splenectomy. NS, non-significant. |
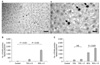 | Fig. 2Liver regeneration index measured by the number of 5-bromo-2'-deoxyuridine (BrdU)-positive hepatocytes. (A) Photomicrographs showed liver sections stained for BrdU (A-a, arrows) and hepatocytic nuclei. Scale bar 400 mm (A) and 50 mm (a). (B) Liver regeneration indexes were significantly different according to the amount of liver resection (P < 0.001). (C) The differences between subgroups according to the splenectomy were significant only in 90% hepatectomy group (P < 0.001). 70 (90)% ± S, 70 (90)% hepatectomy with and without splenectomy; 70 (90)%, 70 (90)% hepatectomy without splenectomy; 70 (90)%±S, 70 (90)% hepatectomy with splenectomy; NS, non-significant. |
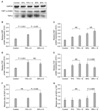 | Fig. 3Western blot analysis of hepatic growth factor (HGF) and transforming growth factor-β (TGF-β). (A) The intensities of α-chain of HGF and TGF-β were calculated by using densitometry. (B) Relative protein expressions of HGF were significantly higher in hepatectomized groups than the control group, and it was higher in 90% hepatectomy group than in 70% hepatectomy group. (C) However, those were not significant between subgroups according to the splenectomy. (D) The expressions of TGF-β were also significantly higher in hepatectomized groups. However, the difference was not significant between 70% and 90% hepatectomy groups. (E) Subgroup analysis showed that TGF-β expressions were significantly lower in the splenectomy group, in 90% hepatectomy groups but not in 70% hepatectomy groups. (F) To show the relative balance between HGF and TGF-β, the relative ratio of HGF to TGF-β was also analyzed. Difference of the ratio was not significant between the control group and the 70% hepatectony group but it was significantly higher in the 90% group. (G) The subgroup analysis showed that splenectomy significantly increased HGF to TGF-β ratio only in the 90% hepatectomy group. Among subgroups without splenectomy (bars marked with asterisks), there were no significant differences. Values are presented as mean ± standard error of the mean. 70 (90)% ± S, 70 (90)% hepatectomy with and without splenectomy; 70 (90)%, 70 (90)% hepatectomy without splenectomy; 70 (90)% + S, 70 (90)% hepatectomy with splenectomy; NS, non-significant. |
ACKNOWLEDGEMENTS
This research was supported by a grant (KHU-20091438) from the Kyung Hee University Program for the Young Researcher in Medical Science.
References
1. Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007. 356:1545–1559.
2. Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005. 5:2605–2610.
3. Marubashi S, Dono K, Miyamoto A, Takeda Y, Nagano H, Umeshita K, et al. Impact of graft size on postoperative thrombocytopenia in living donor liver transplant. Arch Surg. 2007. 142:1054–1058.
4. Saner F, Sotiropoulos GC, Radtke A, Malago M, Broelsch CE, Herget-Rosenthal S. Small-for-size syndrome after living-donor liver transplantation treated by "portal vein wrapping" and single plasmapheresis. Transplantation. 2005. 79:625.
5. Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, et al. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005. 5:1397–1404.
6. Umeda Y, Yagi T, Sadamori H, Matsukawa H, Matsuda H, Shinoura S, et al. Effects of prophylactic splenic artery modulation on portal overperfusion and liver regeneration in small-for-size graft. Transplantation. 2008. 86:673–680.
7. Arakawa Y, Shimada M, Uchiyama H, Ikegami T, Yoshizumi T, Imura S, et al. Beneficial effects of splenectomy on massive hepatectomy model in rats. Hepatol Res. 2009. 39:391–397.
8. Sugawara Y, Yamamoto J, Shimada K, Yamasaki S, Kosuge T, Takayama T, et al. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000. 190:446–450.
9. Eipel C, Glanemann M, Nuessler AK, Menger MD, Neuhaus P, Vollmar B. Ischemic preconditioning impairs liver regeneration in extended reduced-size livers. Ann Surg. 2005. 241:477–484.
10. Kelly DM, Demetris AJ, Fung JJ, Marcos A, Zhu Y, Subbotin V, et al. Porcine partial liver transplantation: a novel model of the "small-for-size" liver graft. Liver Transpl. 2004. 10:253–263.
11. Eipel C, Abshagen K, Ritter J, Cantré D, Menger MD, Vollmar B. Splenectomy improves survival by increasing arterial blood supply in a rat model of reduced-size liver. Transpl Int. 2010. 23:998–1007.
12. Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931. 12:186–202.
13. Gaub J, Iversen J. Rat liver regeneration after 90% partial hepatectomy. Hepatology. 1984. 4:902–904.
14. Bucher NL. Jirtle RL, editor. Liver regeneration then and now. Liver regeneration and carcinogenesis: molecular and cellular mechanisms. 1995. San Diego: Academic Press;1–25.
15. Kim J, Yi NJ, Shin WY, Kim T, Lee KU, Suh KS. Platelet transfusion can be related to liver regeneration after living donor liver transplantation. World J Surg. 2010. 34:1052–1058.
16. Maetani Y, Itoh K, Egawa H, Shibata T, Ametani F, Kubo T, et al. Factors influencing liver regeneration following living-donor liver transplantation of the right hepatic lobe. Transplantation. 2003. 75:97–102.
17. Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997. 276:60–66.
18. Ichikawa T, Zhang YQ, Kogure K, Hasegawa Y, Takagi H, Mori M, et al. Transforming growth factor beta and activin tonically inhibit DNA synthesis in the rat liver. Hepatology. 2001. 34:918–925.
19. Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010. 176:2–13.
20. Ueda S, Yamanoi A, Hishikawa Y, Dhar DK, Tachibana M, Nagasue N. Transforming growth factor-beta1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab Invest. 2003. 83:1595–1603.
21. Ito K, Ozasa H, Horikawa S. Effects of prior splenectomy on remnant liver after partial hepatectomy with Pringle maneuver in rats. Liver Int. 2005. 25:438–444.
22. Ito K, Ozasa H, Noda Y, Horikawa S. Splenic artery ligation ameliorates hepatic ischemia and reperfusion injury in rats. Liver Int. 2006. 26:254–260.
23. Glanemann M, Eipel C, Nussler AK, Vollmar B, Neuhaus P. Hyperperfusion syndrome in small-for-size livers. Eur Surg Res. 2005. 37:335–341.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download